Hereditary Angioedema Intellia Therapeutics
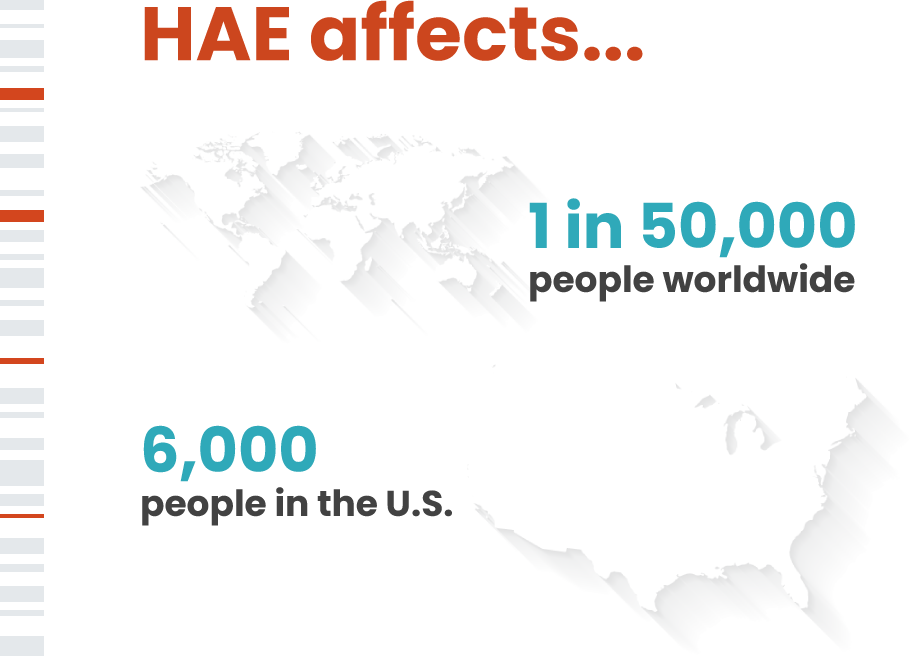
Hereditary Angioedema Intellia Therapeutics Hereditary angioedema (hae) is a rare but serious, genetic condition associated with frequent episodes or attacks of painful swelling in various parts of the body. learn more from intellia therapeutics. Hereditary angioedema is a rare genetic disease that leads to severe and unpredictable swelling attacks. kellie kolb, palak sharma, and zachary dymek (all of intellia therapeutics) for.

Hereditary Angioedema Intellia Therapeutics Intellia’s multi national phase 1 2 study is evaluating the safety, tolerability, pharmacokinetics and pharmacodynamics of ntla 2002 in adults with type i or type ii hereditary angioedema (hae). this includes the measurement of plasma kallikrein protein levels and clinical activity as determined by hae attack rate measures. Intellia’s global phase 1 2 study is evaluating the safety, tolerability, pharmacokinetics and pharmacodynamics of ntla 2002 in adults with type i or type ii hereditary angioedema (hae). this includes the measurement of plasma kallikrein protein levels and activity, as well as hae attack rate. Intellia’s ongoing phase 1 2 study is evaluating the safety and activity of ntla 2002 in adults with type i or type ii hereditary angioedema (hae). the phase 1 2 is an international, open label study designed to identify a dose level of ntla 2002 for further evaluation in a phase 3 study. Intellia therapeutics announces publication of positive interim phase 1 data for ntla 2002 in patients with hereditary angioedema in the new england journal of medicine. cambridge, mass., jan. 31.

Comments are closed.