How Many Valence Electrons Does Copper Cu Have
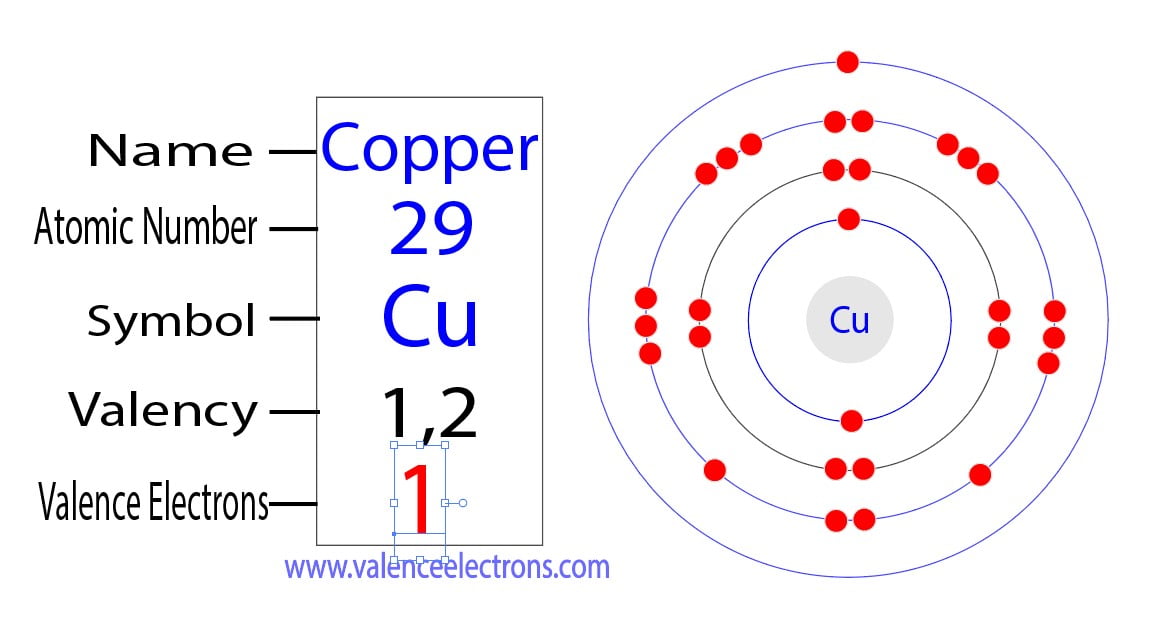
How To Find The Valence Electrons For Copper Cu For this, copper ion (cu ) has a total of eighteen valence electrons. again, the copper atom donates an electron in the 4s orbital and an electron in the 3d orbital to convert copper ion (cu 2 ). cu – 2e – → cu 2 . here, the electron configuration of copper ion (cu 2 ) is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 9. Valence electrons: for main group elements (i.e s block and p block elements), the valence electrons are the electrons present in the outermost orbit. but for most of the transition and inner transition elements, the valence electrons are the electrons present in the shells outside the noble gas core.

How To Find The Valence Electrons For Copper Cu Youtube To find the number of valence electrons for copper (cu)we need to look at its electron configuration. this is necessary because cu is a transition metal (d. Phosphorus valence electrons; how many valence electrons does copper have? copper has the atomic number 29 and the representative symbol as cu. in composition, copper has the structure as a soft, malleable metal. the color of freshly exposed copper generally remains as orange in color. copper is one of the most highly conductive medium of. The valence electron configuration of cu c u is 4sx1 3dx10 4 s x 1 3 d x 10. according to this the answer is 11 11, but shouldn't it be 1 1 since the d shell is already filled up? yes, copper only has 1 valence electron. remember: valence electrons only include the electrons in the highest energy (n) shell. The electron configuration for copper ion (cu2 ), is shown here as 1s2 2s2 2p6 3s2 3p6 3d9.this electron configuration shows that the copper ion has three shells, and the last shell contains seventeen electrons.in this example, the valence electrons for copper ion (cu2 ) are seventeen.
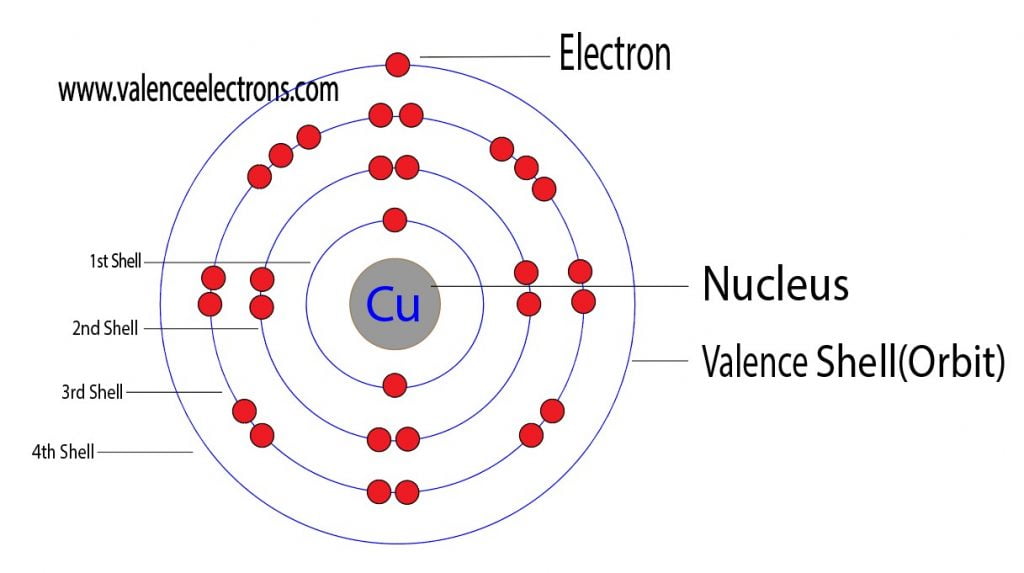
How Many Valence Electrons Does Copper Cu Have The valence electron configuration of cu c u is 4sx1 3dx10 4 s x 1 3 d x 10. according to this the answer is 11 11, but shouldn't it be 1 1 since the d shell is already filled up? yes, copper only has 1 valence electron. remember: valence electrons only include the electrons in the highest energy (n) shell. The electron configuration for copper ion (cu2 ), is shown here as 1s2 2s2 2p6 3s2 3p6 3d9.this electron configuration shows that the copper ion has three shells, and the last shell contains seventeen electrons.in this example, the valence electrons for copper ion (cu2 ) are seventeen. Video: cu, cu , and cu2 electron configuration notation. in writing the electron configuration for copper the first two electrons will go in the 1s orbital. since 1s can only hold two electrons the next 2 electrons for copper go in the 2s orbital. the next six electrons will go in the 2p orbital. the p orbital can hold up to six electrons. Updated on may 19, 2024. you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that can be derived by looking at the groups (columns) of the periodic table. while these are the most common valences, the real behavior of electrons is less simple.
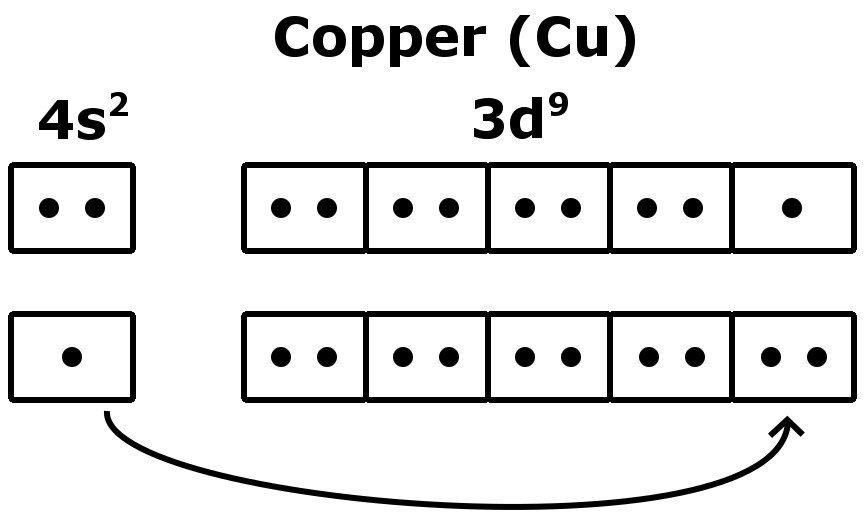
Copper Electron Configuration Cu With Orbital Diagram Video: cu, cu , and cu2 electron configuration notation. in writing the electron configuration for copper the first two electrons will go in the 1s orbital. since 1s can only hold two electrons the next 2 electrons for copper go in the 2s orbital. the next six electrons will go in the 2p orbital. the p orbital can hold up to six electrons. Updated on may 19, 2024. you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that can be derived by looking at the groups (columns) of the periodic table. while these are the most common valences, the real behavior of electrons is less simple.

Comments are closed.