How To Balance Cu No3 2 Ki Cui2 Kno3 Youtube

How To Balance Cu No3 2 Ki Cui2 Kno3 Youtube In this video we'll balance the equation cu(no3)2 ki = cui2 kno3 and provide the correct coefficients for each compound.to balance cu(no3)2 ki = cui2. There are three main steps for writing the net ionic equation for k2s cu(no3)2 = kno3 cus (potassium sulfide copper (ii) nitrate). first, we balance th.
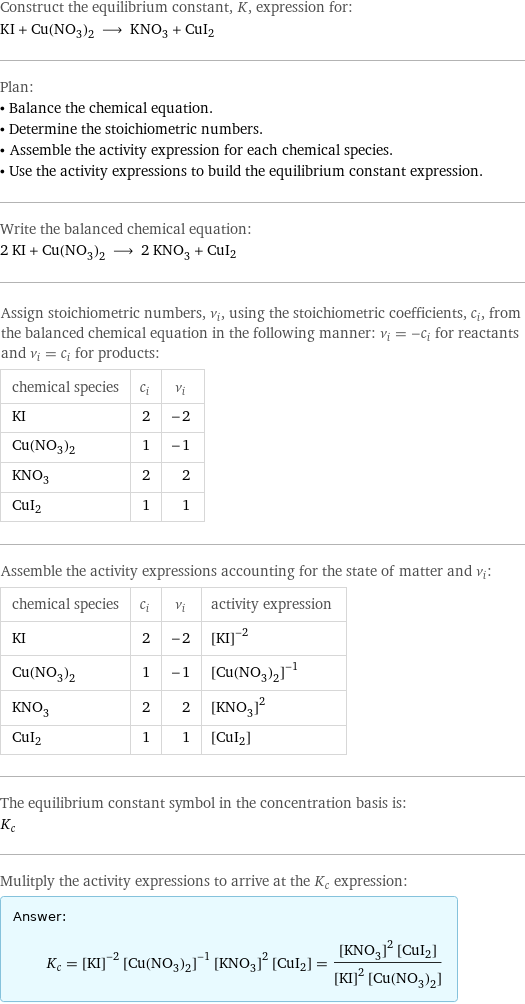
Ki Cu No3 2 Kno3 Cui2 There are three main steps for writing the net ionic equation for k2co3 cu(no3)2 = cuco3 kno3 (potassium carbonate copper (ii) chloride). first, we bal. Balancing step by step using the inspection method. let's balance this equation using the inspection method. first, we set all coefficients to 1: 1 cu (no 3) 2 1 ki = 1 cui 2 1 kno 3. for each element, we check if the number of atoms is balanced on both sides of the equation. cu is balanced: 1 atom in reagents and 1 atom in products. To calculate the limiting reagent in cu(no3)2 ki = kno3 cui2 you must first find the stoichiometric mole ratios of each compound. this can be done by using our chemical equation balancer and taking the coefficients of the balanced equation or by entering it into our stoichiometry calculator. Step 4: substitute coefficients and verify result. count the number of atoms of each element on each side of the equation and verify that all elements and electrons (if there are charges ions) are balanced. 2 cu (no3)2 4 ki = 2 cui 4 kno3 i2. reactants.

Comments are closed.