How To Calculate Mass Percent Of A Solution

Question Video Identifying The Equation Used To Calculate The Calculate the mass percent. now that the equation is filled in, simply solve to calculate the mass percent. divide the mass of the chemical by the total mass of the compound and multiply by 100. this will give you the mass percent of the chemical. example 1: (5 105) x 100 = 0.04761 x 100 = 4.761%. The mass mass percent (% m m) is defined as the mass of a solute divided by the mass of a solution times 100: %m m = massofsolute massofsolution × 100%. mass of solution = mass of solute mass solvent. if you can measure the masses of the solute and the solution, determining the mass mass percent is easy. each mass must be expressed in the.

How To Calculate Mass Percent Of Solute And Solvent Of Solution In this video we will discuss solution composition. more specifically we will discuss one way of looking at solution composition called mass percent. we wi. Aug 10, 2014. the percentage concentration of any solution is most commonly expressed as mass percent: mass % of any component of the solution =. (mass of the component in the solution total mass of the solution) x 100. other methods are: volume percentage: volume % of a component =. (volume of the component total volume of the solution) x. Set up your equation so the concentration c = mass of the solute total mass of the solution. plug in your values and solve the equation to find the concentration of your solution. [6] in our example, c = (10 g) (1,210 g) = 0.00826. 4. multiply your answer by 100 if you want to find the percent concentration. Percent concentration by mass is defined as the mass of solute divided by the total mass of the solution and multiplied by 100%. so, c% = msolute msolution ⋅ 100%, where. msolution = msolvent msolute. there are two ways to change a solution's concentration by mass. adding more solute making the solution more concentrated;.
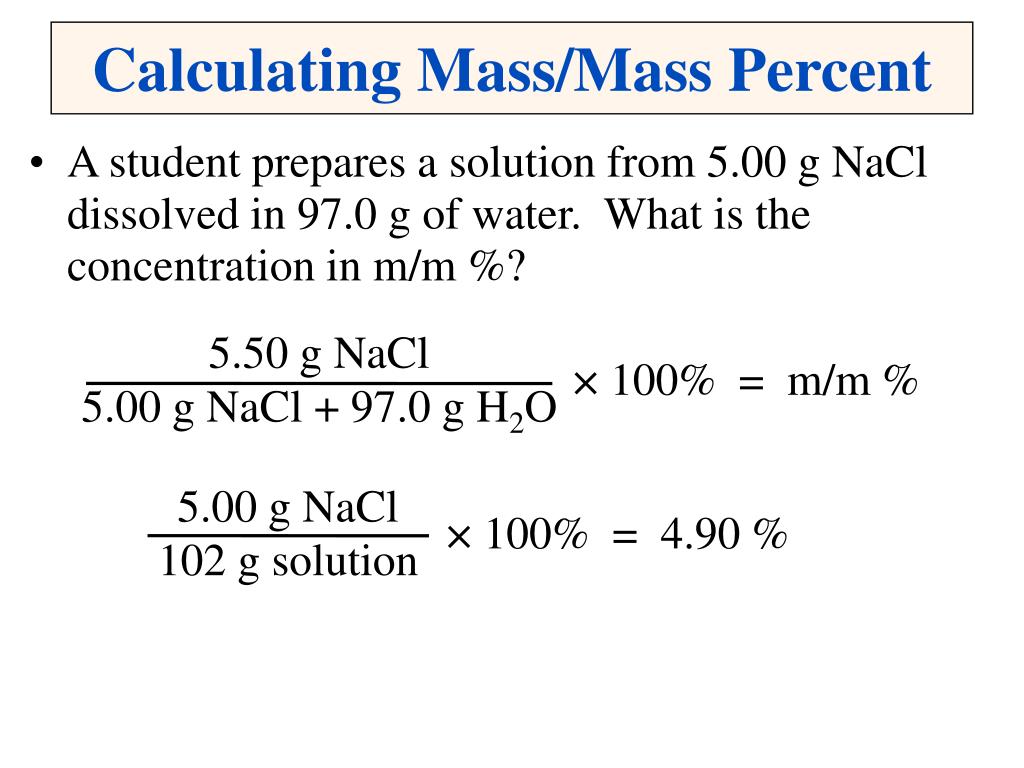
Ppt Solutions Powerpoint Presentation Free Download Id 269800 Set up your equation so the concentration c = mass of the solute total mass of the solution. plug in your values and solve the equation to find the concentration of your solution. [6] in our example, c = (10 g) (1,210 g) = 0.00826. 4. multiply your answer by 100 if you want to find the percent concentration. Percent concentration by mass is defined as the mass of solute divided by the total mass of the solution and multiplied by 100%. so, c% = msolute msolution ⋅ 100%, where. msolution = msolvent msolute. there are two ways to change a solution's concentration by mass. adding more solute making the solution more concentrated;. Mass percent concentration (wt%) is one of the types of percentage concentration. it is defined as the ratio of the solute's mass to the solution's total mass. then it's multiplied by 100% to arrive at a percentage. written as a formula, that's: wt% = m₁ m₂ × 100%. where: m₁ – mass of solute [g]; and; m₂ – total mass of solution [g]. This is a whiteboard animation tutorial on how to solve mass percent calculations for solutions.please support me on patreon: patreon ketzbook.

Comments are closed.