How To Determine The 4 Quantum Numbers From An Element Or A Valence Electron

Question Video Determining The Quantum Numbers That Represent An Step 1: find the element on the periodic table. step 2: determine n and l by identifying the period # and the block that the element is located in. step 3: determine ml by labeling the block from. This video shows you how to identify or determine the 4 quantum numbers (n, l, ml, and ms) from an element or valence electron. this video provides 3 exampl.

Quantum Numbers For 7th Electron The probability distributions are given by the secondary quantum number l and by the magnetic quantum number m l. the now outdated solar system model of the atom allows us to visualize the meaning of the potential energy levels. the main energy levels, also called shells, are given by the main quantum number n. First electron. n = 1 ℓ = 0 m ℓ = 0 m s = ½. the first electron in helium has exactly the same four quantum number of the first electron in hydrogen. however, helium has two electrons. so we "build up" from the previous electrons by adding one more. second electron. n = 1 ℓ = 0 m ℓ = 0 m s = ½. How to determine the 4 quantum numbers from an element or a valence electron. Bonus example #1: assign a correct set of four quantum numbers for the valence electron in a sodium atom. solution: 1) sodium has a total of eleven electrons and one of them is the sole valence electron that sodium has. i propose to assign all ten sets of quantum numbers and build up to the eleventh set, which will be the answer to the question.
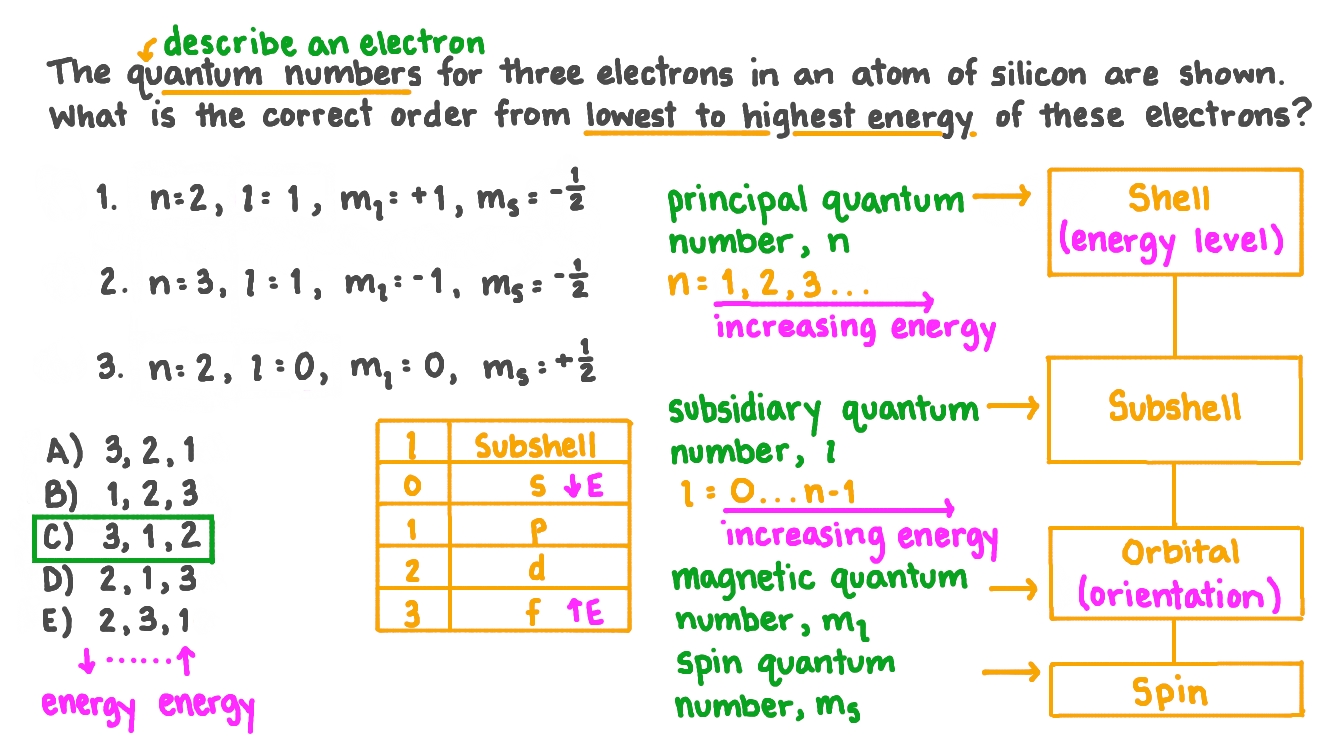
Question Video Ranking Three Electrons From Lowest To Highest Energy How to determine the 4 quantum numbers from an element or a valence electron. Bonus example #1: assign a correct set of four quantum numbers for the valence electron in a sodium atom. solution: 1) sodium has a total of eleven electrons and one of them is the sole valence electron that sodium has. i propose to assign all ten sets of quantum numbers and build up to the eleventh set, which will be the answer to the question. In atoms, there are a total of four quantum numbers: the principal quantum number ( n ), the orbital angular momentum quantum number ( l ), the magnetic quantum number ( ml ), and the electron spin quantum number ( ms ). the principal quantum number, n n, describes the energy of an electron and the most probable distance of the electron from. In the quantum mechanical model of an atom, the state of an electron is described by four quantum numbers, not just the one predicted by bohr. the first quantum number is called the principal quantum number (n). the principal quantum number largely determines the energy of an electron. electrons in the same atom that have the same principal.

Quantum Numbers Diagram In atoms, there are a total of four quantum numbers: the principal quantum number ( n ), the orbital angular momentum quantum number ( l ), the magnetic quantum number ( ml ), and the electron spin quantum number ( ms ). the principal quantum number, n n, describes the energy of an electron and the most probable distance of the electron from. In the quantum mechanical model of an atom, the state of an electron is described by four quantum numbers, not just the one predicted by bohr. the first quantum number is called the principal quantum number (n). the principal quantum number largely determines the energy of an electron. electrons in the same atom that have the same principal.

Comments are closed.