How To Find Protons Electrons For The O 2 Oxide Ion

How To Find Protons Electrons For The O 2 Oxide Ion Youtube In this video we’ll use the periodic table and a few simple rules to find the number of protons and electrons for the oxide ion (o2 ). from the periodic tabl. In this video we will write the electron configuration for o 2 , the oxide ion. we’ll also look at why oxygen forms a 2 ion and how the electron configurati.
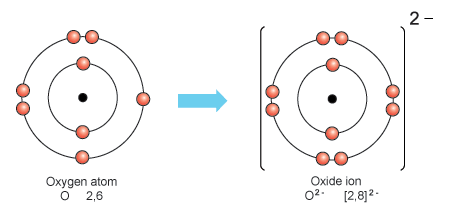
Draw The Diagram Representing The Atomic Structure Of The Oxide Ion In this video we’ll use the periodic table and a few simple rules to find the protons, electrons, and neutrons for ions. from the periodic table we can find. Example 2.5.2. an ion with a 2 charge has 18 electrons. determine the identity of the ion. solution. if an ion has a 2 charge then it must have lost electrons to form the cation. if the ion has 18 electrons and the atom lost 2 to form the ion, then the neutral atom contained 20 electrons. The answer is the protons and the electrons. the protons have a positive charge, while electrons are negatively charged. if you remove one or more electrons from the atom, you will receive a positive ion called a cation as you will leave more protons than electrons. similarly, by adding electrons, you will gain a negative ion, called an anion. To calculate the remaining number of electrons, you subtract the amount of extra charge from the atomic number. in the case of a positive ion, there are more protons than electrons. for example, ca 2 has a 2 charge so it has lost 2 electrons from the neutral state. calcium’s atomic number is 20, therefore the ion has 18 electrons.
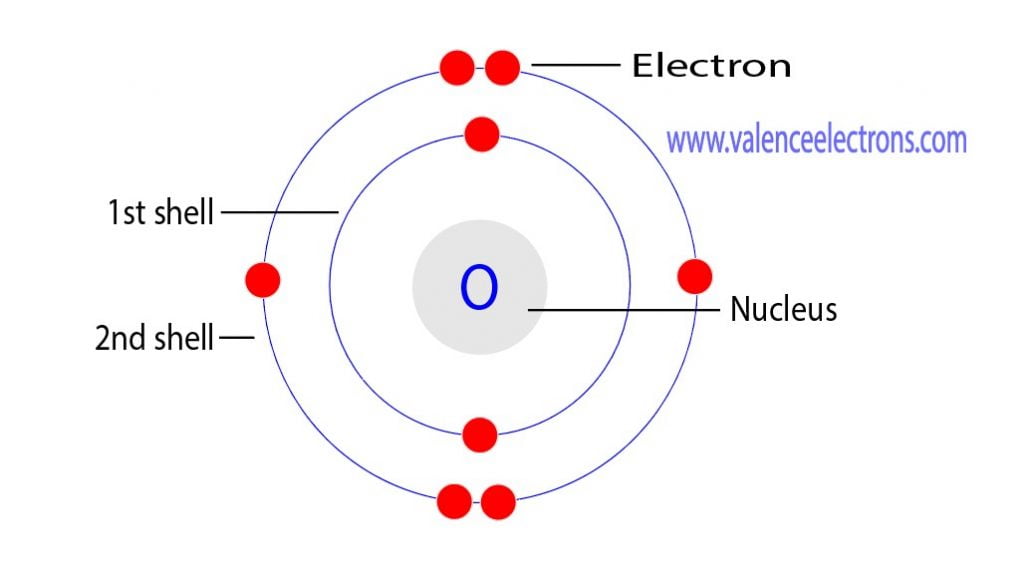
Protons Neutrons Electrons For Oxygen O O2вђ The answer is the protons and the electrons. the protons have a positive charge, while electrons are negatively charged. if you remove one or more electrons from the atom, you will receive a positive ion called a cation as you will leave more protons than electrons. similarly, by adding electrons, you will gain a negative ion, called an anion. To calculate the remaining number of electrons, you subtract the amount of extra charge from the atomic number. in the case of a positive ion, there are more protons than electrons. for example, ca 2 has a 2 charge so it has lost 2 electrons from the neutral state. calcium’s atomic number is 20, therefore the ion has 18 electrons. Oxygen has the atomic number 8, which means the nuclei of its atoms have 8 protons. a neutral oxygen atom as also has 8 electrons. the oxide anion has a charge of 2 . this means that it has gained two electrons from another element, such as sodium or magnesium. all oxide anions, "o"^(2 )", have 8 protons and 10 electrons. To get a 2 charge, the ion had to lose two electrons. a magnesium ion therefore has 10 electrons. (b) since n 3− is an anion, its name is the root name of the element with the suffix ide. this would make it a nitride ion. nitrogen atoms contain 7 protons in their nucleus. to get a 3 charge, the ion had to gain three electrons. a nitride.

O 2 Electron Configuration Oxide Ion Youtube Oxygen has the atomic number 8, which means the nuclei of its atoms have 8 protons. a neutral oxygen atom as also has 8 electrons. the oxide anion has a charge of 2 . this means that it has gained two electrons from another element, such as sodium or magnesium. all oxide anions, "o"^(2 )", have 8 protons and 10 electrons. To get a 2 charge, the ion had to lose two electrons. a magnesium ion therefore has 10 electrons. (b) since n 3− is an anion, its name is the root name of the element with the suffix ide. this would make it a nitride ion. nitrogen atoms contain 7 protons in their nucleus. to get a 3 charge, the ion had to gain three electrons. a nitride.

Lewis Structure Of O 2 Oxide Ion Youtube

Comments are closed.