How To Find Valence Electrons For Iron Fe Ii Easy Quick
How To Find The Valence Electrons For Iron Fe This video will explain the concept of finding valence electrons of iron (fe) which is a transition element d block elements. in this case the electronic co. 2. find the electron configuration for the element you are examining. once you know an element's electron configuration, finding its number of valence electrons is quite simple (except, of course, for the transition metals.) if you're given the configuration from the get go, you can skip to the next step.

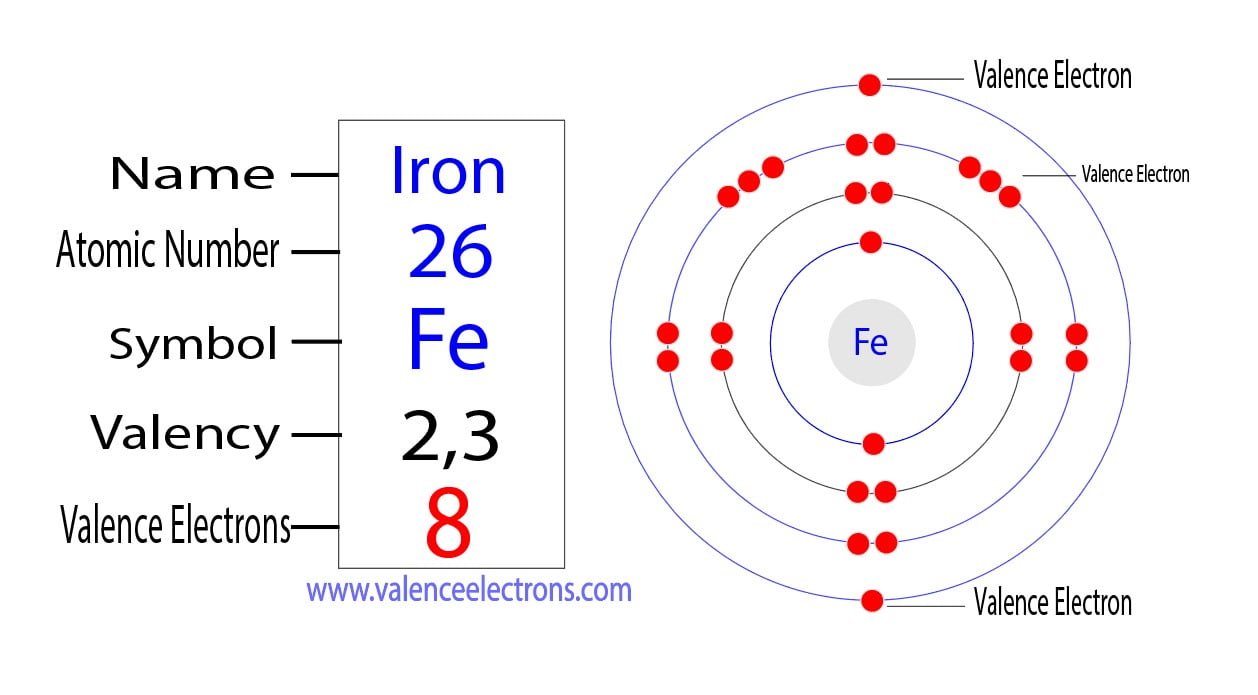
How To Find The Valence Electrons For Iron Fe To find the number of valence electrons for fe (iron) we need to look at its electron configuration. this is necessary because fe is a transition metal (d b. The 4s23d6 electrons. iron thus has 8 valence electrons! easy peasy, once you know the trick! note: just because iron has 8 valence electrons doesn't mean that it will use them all. iron usually uses only two or three of its valence electrons to form compounds. answer link. iron has 8 valence electrons. > this is tricky! you need to have a firm. Valence electrons are those electrons that reside in the outermost shell surrounding an atomic nucleus. valence electrons are of crucial importance because they lend deep insight into an element’s chemical properties: whether it is electronegative or electropositive in nature, or they indicate the bond order of a chemical compound – the number of bonds that can be formed between two atoms. The last shell of iron has two electrons and the d subshell has a total of six electrons. therefore, the valence electrons of iron are eight. valence electrons of iron (fe) the valence electrons determine the element’s properties and participate in forming bonds. iron participates in the formation of bonds through its valence electrons.

Valence Electrons For Fe Iron Youtube Valence electrons are those electrons that reside in the outermost shell surrounding an atomic nucleus. valence electrons are of crucial importance because they lend deep insight into an element’s chemical properties: whether it is electronegative or electropositive in nature, or they indicate the bond order of a chemical compound – the number of bonds that can be formed between two atoms. The last shell of iron has two electrons and the d subshell has a total of six electrons. therefore, the valence electrons of iron are eight. valence electrons of iron (fe) the valence electrons determine the element’s properties and participate in forming bonds. iron participates in the formation of bonds through its valence electrons. Contributors and attributions. 3.10: valence electrons is shared under a cc by nc license and was authored, remixed, and or curated by libretexts. valence electrons are the electrons in the highest occupied principal energy level of an atom. in the second period elements, the two electrons in the 1s sublevel are called inner shell electrons …. 6. you can get the valence electrons in an atom's electronic arrangement by consulting the periodic table: the group 1 atoms have 1 valence electron. the group 2 atoms have 2 valence electrons. the group 3 atoms have 3 valence electrons. the group 4 atoms have 4 valence electrons. the group 5 atoms have 5 valence electrons.

Iron Orbital Notation Contributors and attributions. 3.10: valence electrons is shared under a cc by nc license and was authored, remixed, and or curated by libretexts. valence electrons are the electrons in the highest occupied principal energy level of an atom. in the second period elements, the two electrons in the 1s sublevel are called inner shell electrons …. 6. you can get the valence electrons in an atom's electronic arrangement by consulting the periodic table: the group 1 atoms have 1 valence electron. the group 2 atoms have 2 valence electrons. the group 3 atoms have 3 valence electrons. the group 4 atoms have 4 valence electrons. the group 5 atoms have 5 valence electrons.

Comments are closed.