How To Make Ionic Compounds
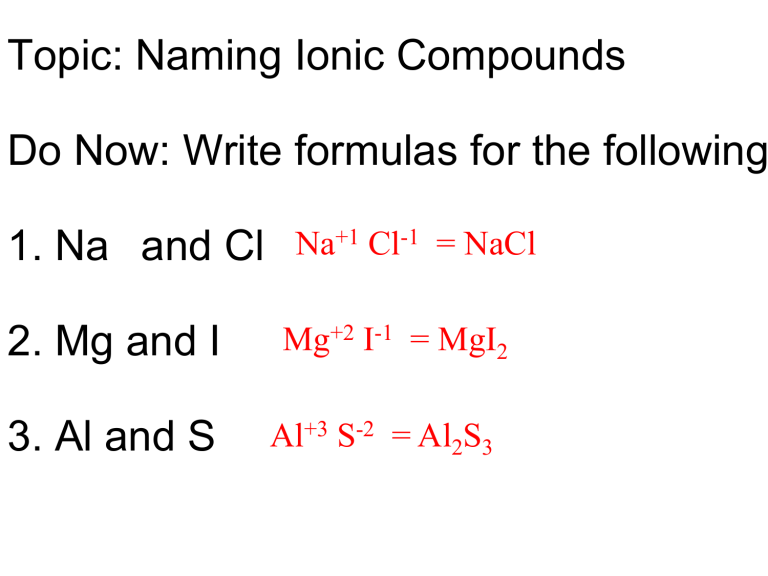
Binary Ionic Compounds Writing Formulas And Naming Compounds Riset Exercise 5.5.2 5.5. 2. write the chemical formula for an ionic compound composed of each pair of ions. the calcium ion and the oxygen ion. the 2 copper ion and the sulfur ion. the 1 copper ion and the sulfur ion. answer a: answer b: answer c: be aware that ionic compounds are empirical formulas and so must be written as the lowest ratio of. This chemistry video tutorial explains how to write chemical formulas of ionic compounds including those with transition metals and polyatomic ions.chemistry.
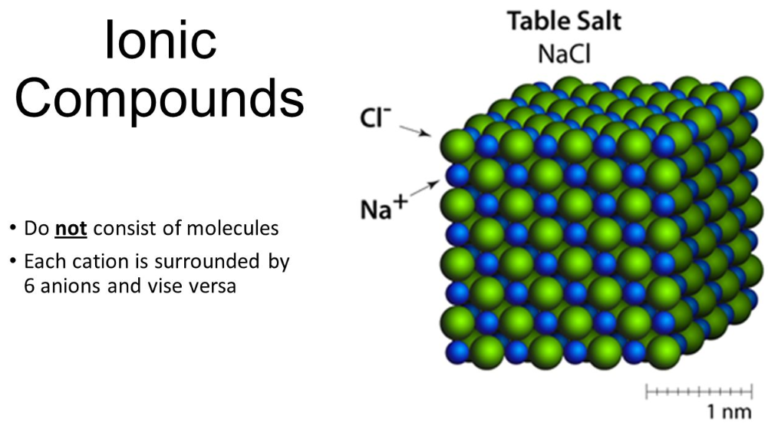
What Is An Ionic Compound Formula And Defination This chemistry video tutorial provides an introduction to writing the formula of an ionic compound that contains transition metals with roman numerals and po. 1. identify a binary compound. the simplest type of ionic compound is made from exactly 2 elements, 1 metal and 1 non metal. their name is always written as 2 element names, plus the ide suffix attached to the second name. [1] examples of simple binary ionic compounds include potassium oxide and sodium phosphide. Key takeaways. ions form when atoms lose or gain electrons. ionic compounds have positive ions and negative ions. ionic formulas balance the total positive and negative charges. ionic compounds have a simple system of naming. groups of atoms can have an overall charge and make ionic compounds. Video transcript. learn for free about math, art, computer programming, economics, physics, chemistry, biology, medicine, finance, history, and more. khan academy is a nonprofit with the mission of providing a free, world class education for anyone, anywhere.

How To Make Ionic Compounds Howcast Key takeaways. ions form when atoms lose or gain electrons. ionic compounds have positive ions and negative ions. ionic formulas balance the total positive and negative charges. ionic compounds have a simple system of naming. groups of atoms can have an overall charge and make ionic compounds. Video transcript. learn for free about math, art, computer programming, economics, physics, chemistry, biology, medicine, finance, history, and more. khan academy is a nonprofit with the mission of providing a free, world class education for anyone, anywhere. First, we need to come up with the correct formula of the ions. in this case, we have li and i –. next, we want to determine how many of each ion we need in the formula of the salt. for this, place the ions next to each other: remember, the idea is that the charges must be balanced making a neutral structure. Learn for free about math, art, computer programming, economics, physics, chemistry, biology, medicine, finance, history, and more. khan academy is a nonprofit with the mission of providing a free, world class education for anyone, anywhere.

Comments are closed.