How To Read A Phase Change Diagram

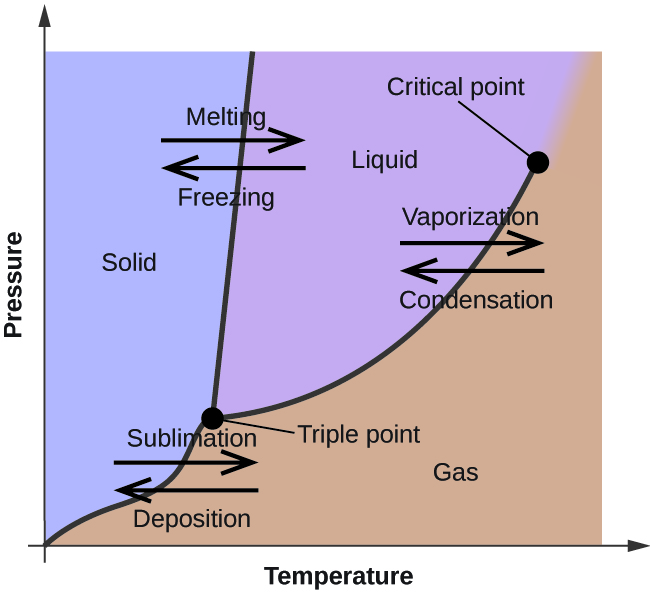
Phase Change Diagrams вђ Overview Examples Expii Phase diagram is a graphical representation of the physical states of a substance under different conditions of temperature and pressure. a typical phase diagram has pressure on the y axis and temperature on the x axis. as we cross the lines or curves on the phase diagram, a phase change occurs. in addition, two states of the substance coexist. In the video here, sal uses a horizontal line through the phase diagram. but, it doesn't have to be horizontal. imagine a vertical line through this diagram for water, choose 100 degrees c. as long as you are at 100 c, you can change the phase by changing the pressure on the system.

Phase Change Diagrams вђ Overview Examples Expii This is a chemistry tutorial video that goes through how to read, analyze, and interpret a phase diagram. there are several examples of different questions y. Three dimensional phase change diagrams plot three thermodynamic variables and show regions of space corresponding to different phases. in this type of diagram, we have a triple line instead of a triple point, and coexistence surfaces instead of coexistence curves. below is a generic 3d diagram plotting temperature, pressure, and specific volume. Solution: when two substances or objects at different temperatures are brought into contact, heat will flow from the warmer one to the cooler. the amount of heat that flows is given by. q = mcsΔt (16.3.1) (16.3.1) q = m c s Δ t. where q is heat, m is mass, cs is the specific heat, and Δ t is the temperature change. Phase diagram. a phase diagram represents the various physical states or phases of matter at different pressures and temperatures. in other words, it summarizes the effect of pressure and temperature on the nature of a substance. a phase diagram is divided into three areas representing the substances’ solid, liquid, and gaseous phases [1 4].
Phase Diagrams Ck 12 Foundation Solution: when two substances or objects at different temperatures are brought into contact, heat will flow from the warmer one to the cooler. the amount of heat that flows is given by. q = mcsΔt (16.3.1) (16.3.1) q = m c s Δ t. where q is heat, m is mass, cs is the specific heat, and Δ t is the temperature change. Phase diagram. a phase diagram represents the various physical states or phases of matter at different pressures and temperatures. in other words, it summarizes the effect of pressure and temperature on the nature of a substance. a phase diagram is divided into three areas representing the substances’ solid, liquid, and gaseous phases [1 4]. Phase diagrams are graphical representations of the different phases and phase transitions of a substance under varying conditions of temperature and pressure. in this book chapter, you will learn how to interpret and construct phase diagrams for pure substances and mixtures, and how to apply them to understand the behavior of matter. this is a useful supplement for general chemistry students. This video explains how simple phase change diagrams are made and how to read them.
:max_bytes(150000):strip_icc()/phase-changes-56a12ddd3df78cf772682e07.png)
Phase Changes Phase diagrams are graphical representations of the different phases and phase transitions of a substance under varying conditions of temperature and pressure. in this book chapter, you will learn how to interpret and construct phase diagrams for pure substances and mixtures, and how to apply them to understand the behavior of matter. this is a useful supplement for general chemistry students. This video explains how simple phase change diagrams are made and how to read them.
Phase Diagrams Chemistry

Comments are closed.