How To Write The Atomic Orbital Diagram For Copper Cu

How To Write The Atomic Orbital Diagram For Copper Cu Youtube To write the orbital diagram for the copper (cu) first we need to write the electron configuration for just . to do that we need to find the number of elect. There are two types of copper ions. the copper atom exhibits cu and cu 2 ions. the copper atom donates an electron in the 4s orbital to form a copper ion(cu ). cu – e – → cu here, the electron configuration of copper ion(cu ) is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10. on the other hand, the copper atom donates an electron in the 4s orbital.
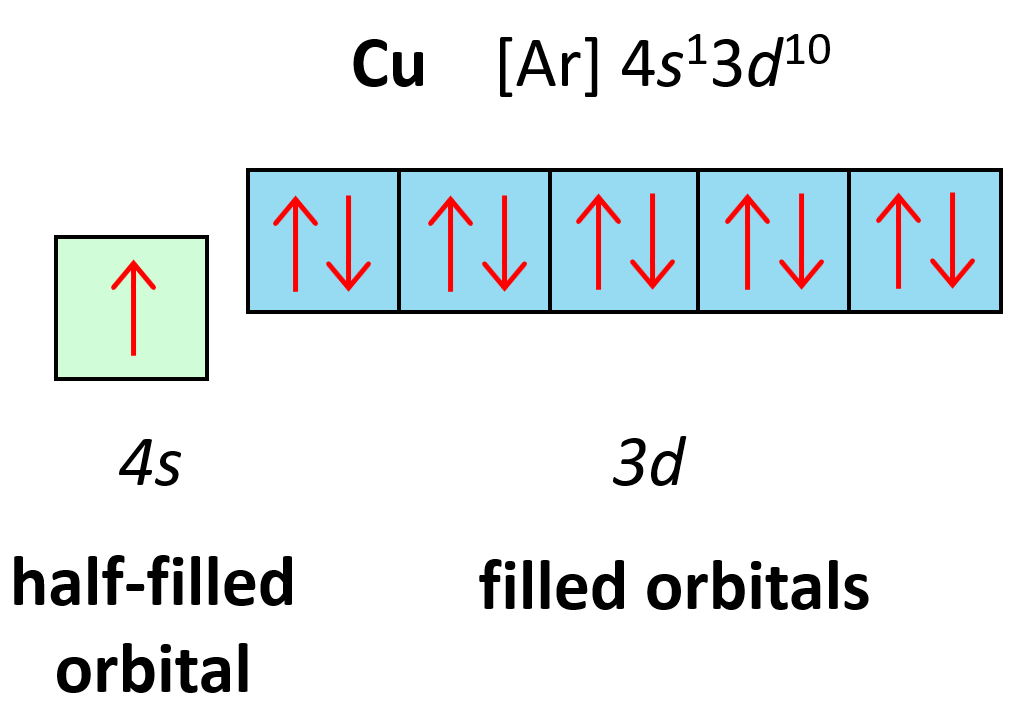
Orbital Diagrams Chemistry Steps Video: cu, cu , and cu2 electron configuration notation. in writing the electron configuration for copper the first two electrons will go in the 1s orbital. since 1s can only hold two electrons the next 2 electrons for copper go in the 2s orbital. the next six electrons will go in the 2p orbital. the p orbital can hold up to six electrons. This diagram shows how the electrons in the copper atom are arranged in different orbitals. orbital is the region of space around the nucleus of an atom where electrons are found. to create an orbital diagram of copper, you first need to know the atomic orbitals and the orbital notation for the copper atom, and also you need to know hund’s. 1s2 2s2 2p6 3s2 3p6 4s1 3d10 is the electron configuration of cu. if the general pattern of filling electron orbitals is followed, then copper’s electron configuration is 1s2 2s2 2p6 3s23p6 4s2 3d9. the only difference is at the end of the configuration that is in the 3d and 4s shells. the atoms of copper have enough electrons which. Writing electron configuration of copper. mr. causey shows you step by step how to write the electron configuration and orbital notation for copper (cu). you.

Comments are closed.