How To Write The Equilibrium Expression For A Chemical Reaction Law
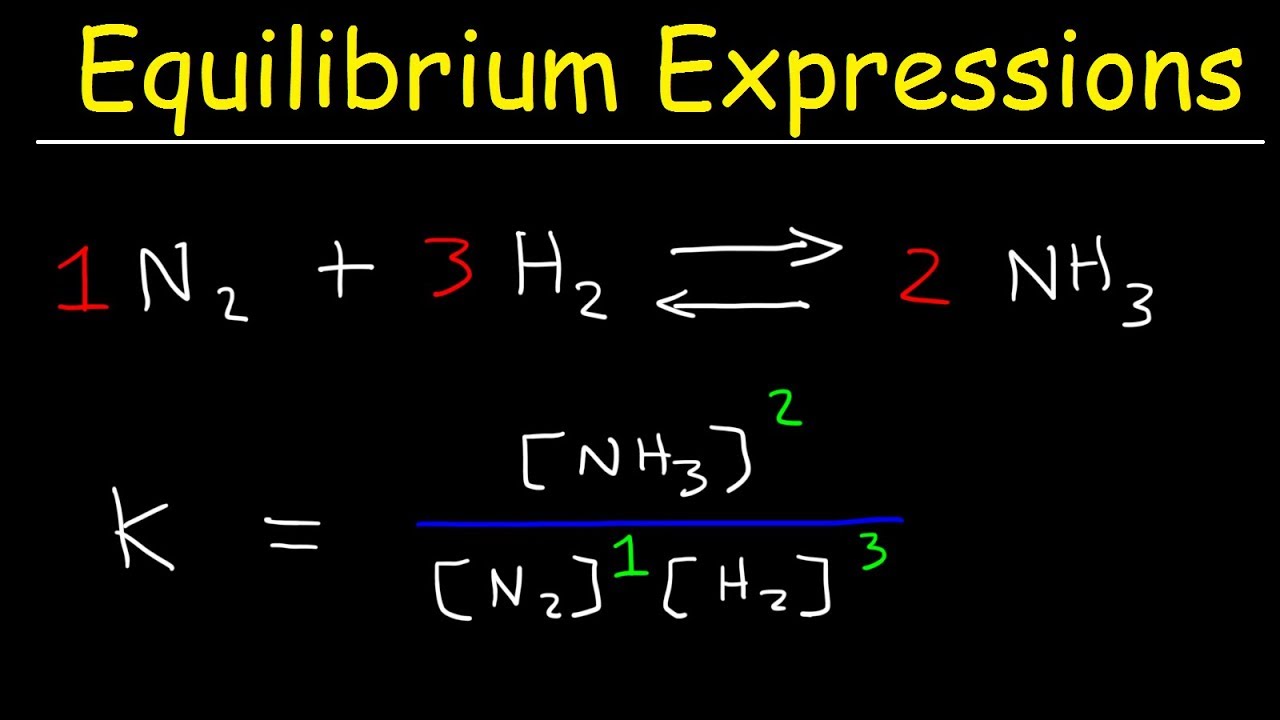
What Is The Equilibrium Constant For The Given Reaction All Answers This relationship is known as the law of mass action and can be stated as follows: k = [c]c[d]d [a]a[b]b. where k is the equilibrium constant for the reaction. equation 15.2.6 is called the equilibrium equation, and the right side of equation 15.2.7 is called the equilibrium constant expression. This chemistry video tutorial explains how to write the equilibrium constant expression for a chemical reaction according the law of mass action principle fo.

How To Write Equilibrium Expressions For Heterogeneous Reactions How to write the expressions for the equilibrium constant and the reaction quotient for different types of chemical reactions? watch this video from khan academy to learn the rules and examples of writing equilibrium expressions. you will also learn how to compare the reaction quotient and the equilibrium constant to predict the direction of the reaction. In each of the heterogeneous processes shown in table 11.4.1, the reactants and products can be in equilibrium (that is, permanently coexist) only when the partial pressure of the gaseous product has the value consistent with the indicated kp. bear in mind also that these kp 's all increase with the temperature. If we write an equation for a gaseous equilibrium in general in the form. aa(g) bb(g) ⇌ cc(g) dd(g) (13.4.1) then the equilibrium constant defined by the equation. kc = [ c ]c[ d ]d [ a ]a[ b ]b (13.4.2) is found to be a constant quantity depending only on the temperature and the nature of the reaction. The denominator of the equilibrium constant expression is the product of the concentrations of the "reactants" raised to a power equal to the coefficient for this component in the balanced equation for the reaction. practice problem 1: write equilibrium constant expressions for the following reactions.
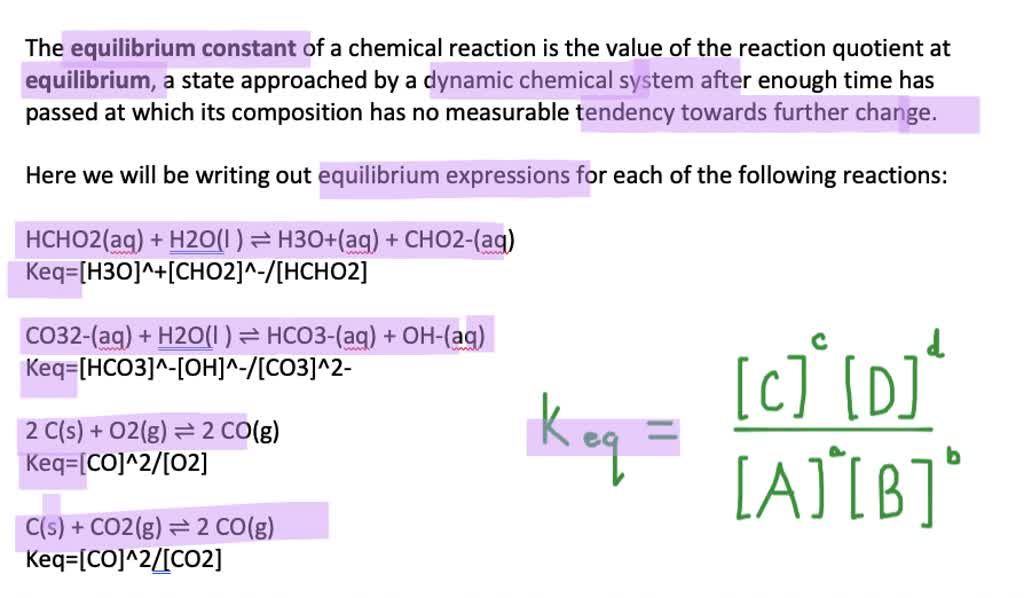
Solved Write An Equilibrium Expression For Each Chemical Equation If we write an equation for a gaseous equilibrium in general in the form. aa(g) bb(g) ⇌ cc(g) dd(g) (13.4.1) then the equilibrium constant defined by the equation. kc = [ c ]c[ d ]d [ a ]a[ b ]b (13.4.2) is found to be a constant quantity depending only on the temperature and the nature of the reaction. The denominator of the equilibrium constant expression is the product of the concentrations of the "reactants" raised to a power equal to the coefficient for this component in the balanced equation for the reaction. practice problem 1: write equilibrium constant expressions for the following reactions. The general equilibrium expression for a reaction: is written as: the brackets " [ ]" represent the concentration of the species (moles per liter or molarity). "a, b, c, and d" represent the coefficients used to balance the equation. the "c" in k c indicates that the value of k is determined using the concentrations of each species. For a general chemical reaction occurring in solution, aa bb ⇄ cc dd. the equilibrium constant, also known as keq, is defined by the following expression: where [a] is the molar concentration of species a at equilibrium, and so forth. the coefficients a, b, c, and d in the chemical equation become exponents in the expression for keq.

Comments are closed.