Identification Of Crystalline Solids Lab Identification Of

Solved Lab Identification Of Crystalline Solids Scenario Chegg Lab: identification of crystalline solids scenario: you are a chemist working in a laboratory where unknown samples are given to you to test and identify. your first assignment is to identify five unknown samples and state their respective type of crystalline. solid. the experimental results are for you to examine and make your final. Question: lab: identification of crystalline solids scenario: you are a chemist working in a laboratory where unknown samples are given to you to test and identify. your first assignment is to identify five unknown samples and state their respective type of crystalline solid. the experimental results are for you to examine and make your final.
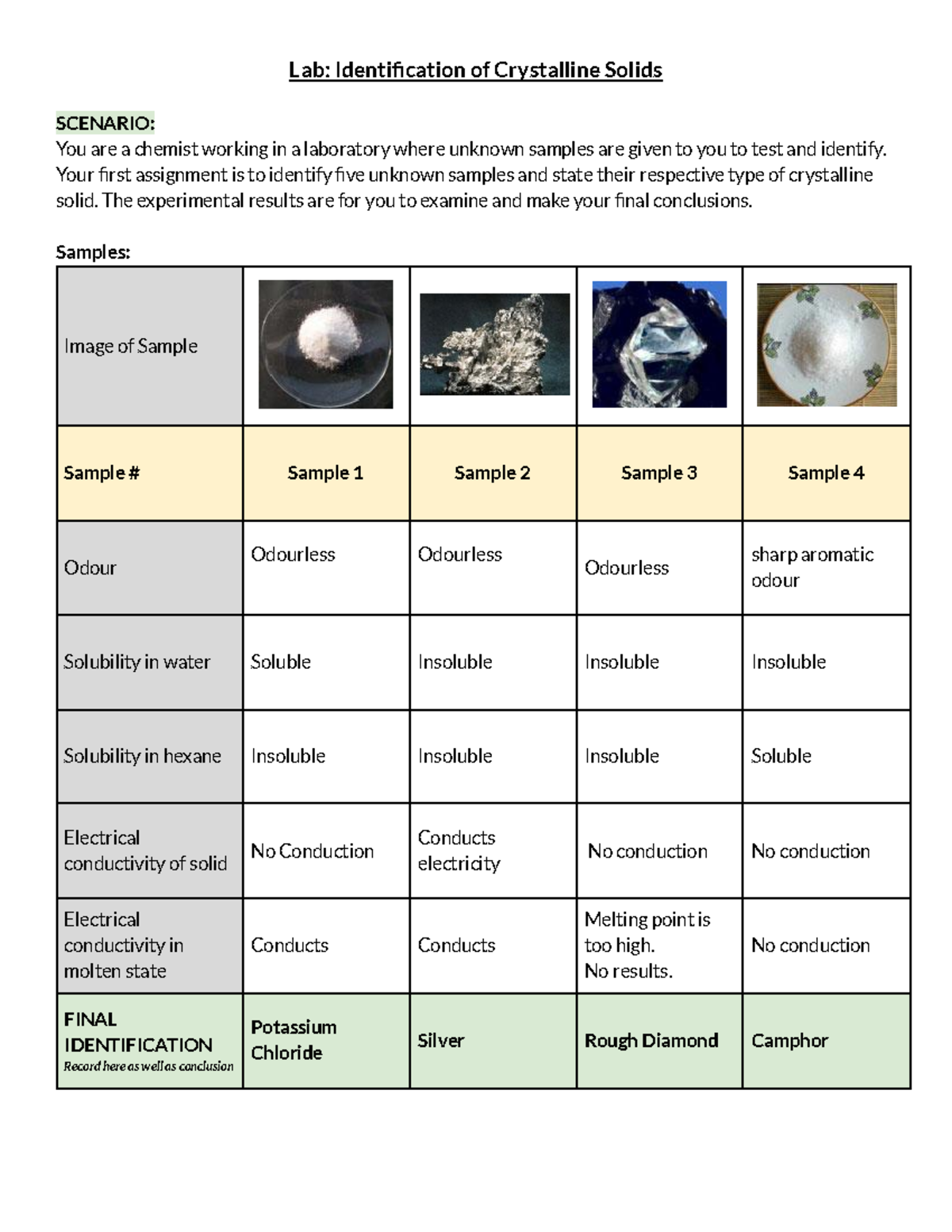
Identification Of Crystalline Solids Lab Identification Of Ilab: identification of crystalline solids scenario: you are a chemist working in a laboratory where unknown samples are given to you to lest and identity. your first assignment is to identity five unknown samples and state their respective type of crystalline solid. the experimental results are for you to examine and make your final conclusions. Crystalline solids, or crystals, have distinctive internal structures that in turn lead to distinctive flat surfaces, or faces. the faces intersect at angles that are characteristic of the substance. when exposed to x rays, each structure also produces a distinctive pattern that can be used to identify the material. Crystalline substances can be described by the types of particles in them and the types of chemical bonding that take place between the particles. there are four types of crystals: (1) ionic, (2) metallic, (3) covalent network, and (4) molecular. properties and several examples of each type are listed in the following table and are described in. Summary. some substances form crystalline solids consisting of particles in a very organized structure; others form amorphous (noncrystalline) solids with an internal structure that is not ordered. the main types of crystalline solids are ionic solids, metallic solids, covalent network solids, and molecular solids.

Solved Lab Activity Identification Of Crystalline Solids Chegg Crystalline substances can be described by the types of particles in them and the types of chemical bonding that take place between the particles. there are four types of crystals: (1) ionic, (2) metallic, (3) covalent network, and (4) molecular. properties and several examples of each type are listed in the following table and are described in. Summary. some substances form crystalline solids consisting of particles in a very organized structure; others form amorphous (noncrystalline) solids with an internal structure that is not ordered. the main types of crystalline solids are ionic solids, metallic solids, covalent network solids, and molecular solids. Ay powder diffraction patterns collected for five different solid phases of a. ties of crystalline forms a–d (usp 1 may 2022) are normalized). (usp 1 may 2022)in addition to the diffraction peaks, an x ray diffraction experi. ent also generates a more or less u. ay 2022) and or the (usp 1 may 2022) equipment, and ot. X ray diffraction techniques are used for the identification of crystalline phases of various materials and the quantitative phase analysis subsequent to the identification. x ray diffraction techniques are superior in elucidating the three dimensional atomic structure of crystalline solids.
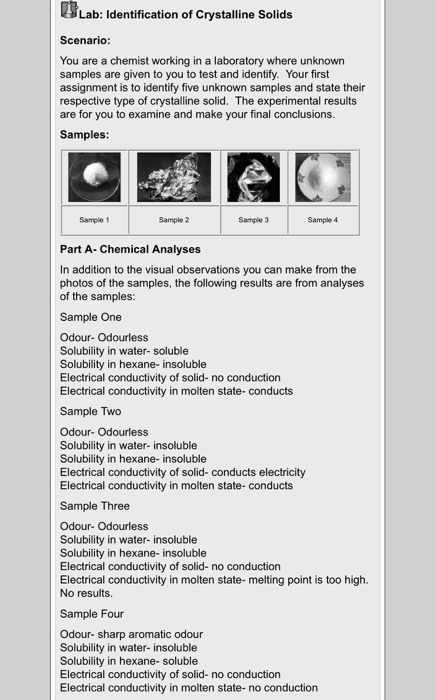
Solved Lab Identification Of Crystalline Solids Scenario Chegg Ay powder diffraction patterns collected for five different solid phases of a. ties of crystalline forms a–d (usp 1 may 2022) are normalized). (usp 1 may 2022)in addition to the diffraction peaks, an x ray diffraction experi. ent also generates a more or less u. ay 2022) and or the (usp 1 may 2022) equipment, and ot. X ray diffraction techniques are used for the identification of crystalline phases of various materials and the quantitative phase analysis subsequent to the identification. x ray diffraction techniques are superior in elucidating the three dimensional atomic structure of crystalline solids.

Comments are closed.