Ind Investigational New Drug Application And Nda
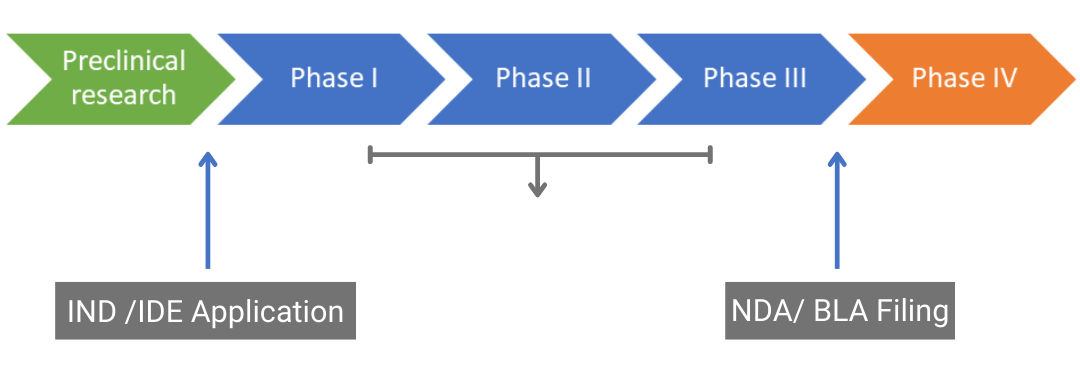
Drug Approval Pathway Ind Investigational New Drug Ide Instructions for sponsors of emergency investigational new drug (eind) applications for antimicrobial products. from the office of antimicrobial products, division of antiviral products. emergency. The nda, on the other hand, is short for “ new drug application “. unlike an ind that comes in during the drug development process and right before the initiation of a clinical trial, an nda signals the end of it. an nda acts as a basis for the fda to control and regulate new drugs in the united states. since 1938, every investigational new.

Ind Investigational New Drug Application And Nda These reports contain information on new drug application (nda), biologic license application (bla), and abbreviated new drug application (anda) approvals. the list also includes a link to. After your ind application has been approved and the clinical trials have been completed, a formal request to market the drug must be made in the form of a new drug application (nda). this application will include all clinical trial data that has been collected through the completed phases carried out following acceptance of the ind. The pages in this section give detailed information about the following types of applications: investigational new drug (ind) current federal law requires that a drug be the subject of an approved. An investigational new drug application (ind) is a request for authorization from the food and drug administration (fda) to administer an investigational drug or biological product to humans. an ind must be authorized prior to interstate shipment and administration of any new drug or biological product that is not the subject of an approved new.
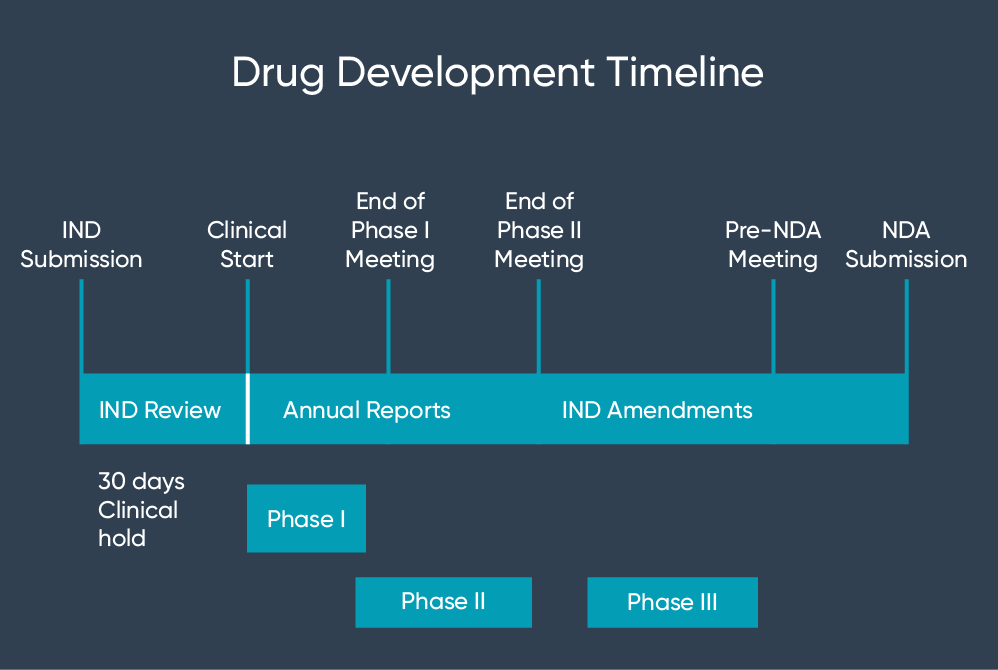
Drug Development Process Map My Xxx Hot Girl The pages in this section give detailed information about the following types of applications: investigational new drug (ind) current federal law requires that a drug be the subject of an approved. An investigational new drug application (ind) is a request for authorization from the food and drug administration (fda) to administer an investigational drug or biological product to humans. an ind must be authorized prior to interstate shipment and administration of any new drug or biological product that is not the subject of an approved new. An investigational new drug offered for import into the united states complies with the requirements of this part if it is subject to an ind that is in effect for it under § 312.40 and: ( 1) the consignee in the united states is the sponsor of the ind; ( 2) the consignee is a qualified investigator named in the ind; or. Those milestone meetings at various stages in development can be critical: the pre ind (investigational new drug application) meeting, the end of phase ii meeting, and the pre nda (new drug application) or pre bla (biologics license application) meeting. a drug development company should tackle those four steps upfront to develop a roadmap and.

The Investigational New Drug Ind And New Drug Application An investigational new drug offered for import into the united states complies with the requirements of this part if it is subject to an ind that is in effect for it under § 312.40 and: ( 1) the consignee in the united states is the sponsor of the ind; ( 2) the consignee is a qualified investigator named in the ind; or. Those milestone meetings at various stages in development can be critical: the pre ind (investigational new drug application) meeting, the end of phase ii meeting, and the pre nda (new drug application) or pre bla (biologics license application) meeting. a drug development company should tackle those four steps upfront to develop a roadmap and.

Comments are closed.