Ind Nda Anda Drug Evolution Process

Ind Nda Anda Drug Evolution Process Abbreviated new drug application (anda) drug application process for nonprescription drugs; investigational new drug (ind) application; new drug application (nda) therapeutic biologics. Description. drug evolution process is a novel concept proposed to develop chemical libraries that have a high probability of finding drugs or drug candidates. it converts biological evolution into chemical evolution. what will you learn. in this course, we will learn about the ind, nda and anda drug evolution process in brief. language: english.
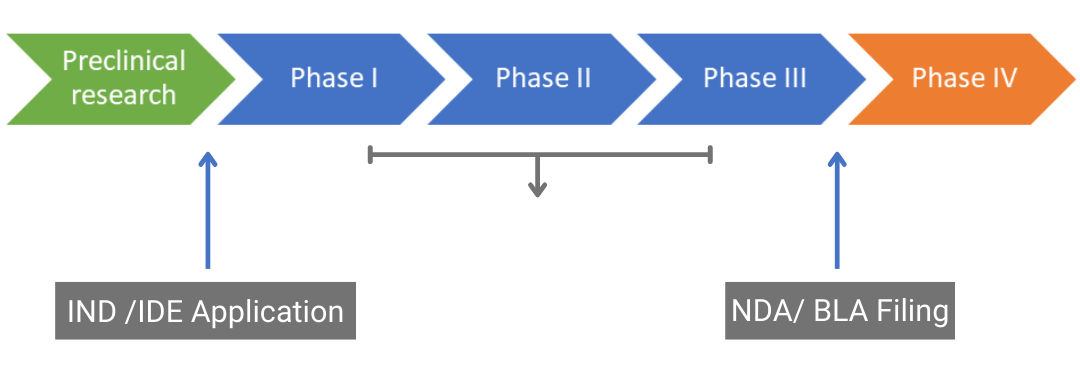
Drug Approval Pathway Ind Investigational New Drug Ide It is common for generic drug applicants to challenge a patent listed in the orange book. in many cases, the brand name drug company patent holder and the anda applicant reach a settlement. Drug review steps simplified. preclinical (animal) testing. an investigational new drug application (ind) outlines what the sponsor of a new drug proposes for human testing in clinical trials. The fda recognizes three primary pathways for the approval of new drugs and abbreviated new drug applications (anda): the 505(b)(1) nda, 505(j) anda, and 505(b)(2) nda. each pathway serves a distinct purpose in the drug approval process. 1. 505(b)(1) nda. the 505(b)(1) nda is a comprehensive application that relies entirely on original data. Ind, nda and anda drug evolution process. the federal food, drug and cosmetics act regulated through title 21 of u.s code of federal regulations, requires a new drug to be approved by fda before legally getting introduced into the market. in india, a new drug may be approved as regulated by schedule y to the rules of drugs and cosmetics act.

Comments are closed.