Intellia Therapeutics Hit 2 Key Milestones In Their Ntla 5001 Program

Intellia Therapeutics Hit 2 Key Milestones In Their Ntla 5001 Program Ntla 2002 for hae: ntla 2002 leverages intellia’s proprietary in vivo lnp delivery technology to knock out the klkb1 gene in the liver with the potential to permanently reduce total plasma kallikrein protein and activity, a key mediator of hae. this investigational approach aims to prevent attacks for people living with hae by providing continuous suppression of plasma kallikrein activity. Ntla 2001 is the first ever investigational in vivo crispr based gene editing therapy cleared to enter late stage clinical development; cambridge, mass., oct. 18, 2023 (globe newswire) intellia therapeutics, inc. (nasdaq:ntla), a leading clinical stage genome editing company focused on developing potentially curative therapies leveraging crispr based technologies, today announced that the u.
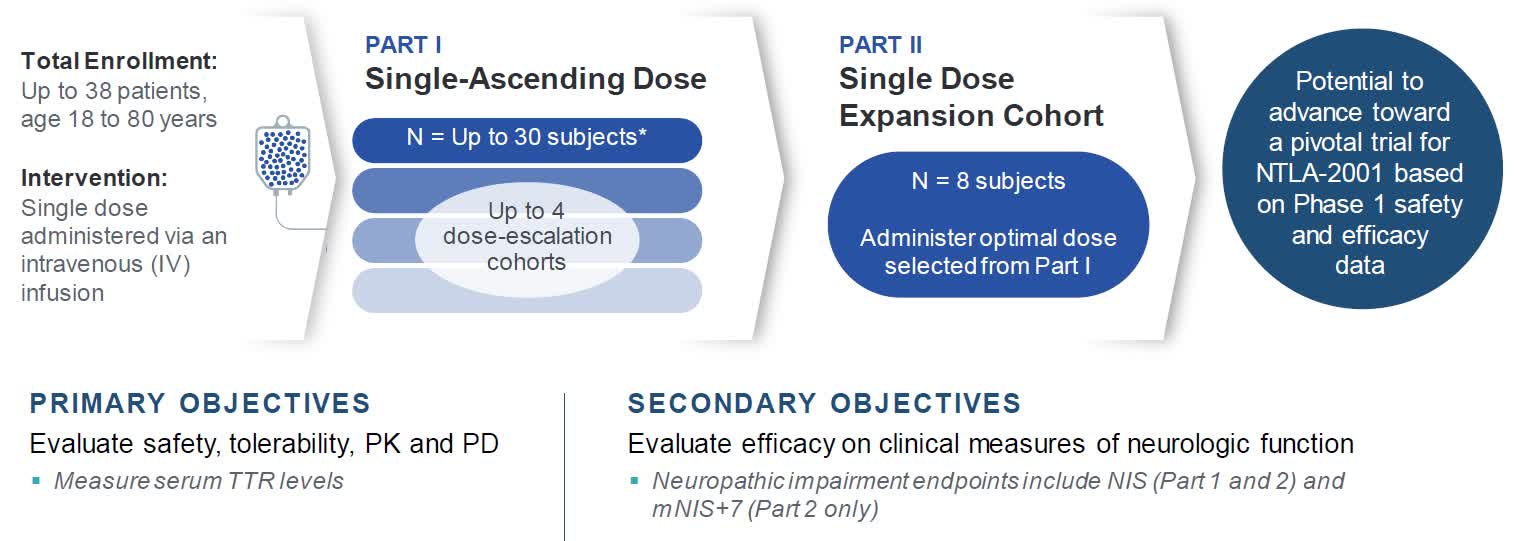
Intellia Therapeutics Nasdaq Ntla Is At A Pivotal Moment Seeking Alpha #ntla, #intelliatherapeutics, #ntla 5001, #crispr, #exvivo, #geneediting, #acutemyeloidleukemia, #orphandrug, #healthwealthin this video we dive into positiv. Intellia therapeutics today announced its strategic priorities through 2026 and key anticipated 2024 milestones that support the company’s mission to transform the lives of patients and bring forth a new era in medicine. intellia anticipates initiating the global pivotal phase 3 trial of ntla 2002 in hae in the second half of 2024, subject to. Ntla 2002 for hae: initiate phase 2 portion of the ongoing ntla 2002 phase 1 2 study in 1h 2023. submit an ind in 1h 2023 to support inclusion of u.s. sites in the phase 2 study of ntla 2002. present additional clinical data from the ongoing first in human study of ntla 2002 in 2023. alpha 1 antitrypsin deficiency (aatd) franchise:. Conclusions and next steps for ntla 5001. proprietary process enables efficient, scalable genome editing. ~99% ko efficiency of target genes; 50 70% in locus insertion of tgtcrs. sequential editing with high viability and potential for safer products. faster t cell expansion with favorable t cell memory phenotype leading to potentially reduced.
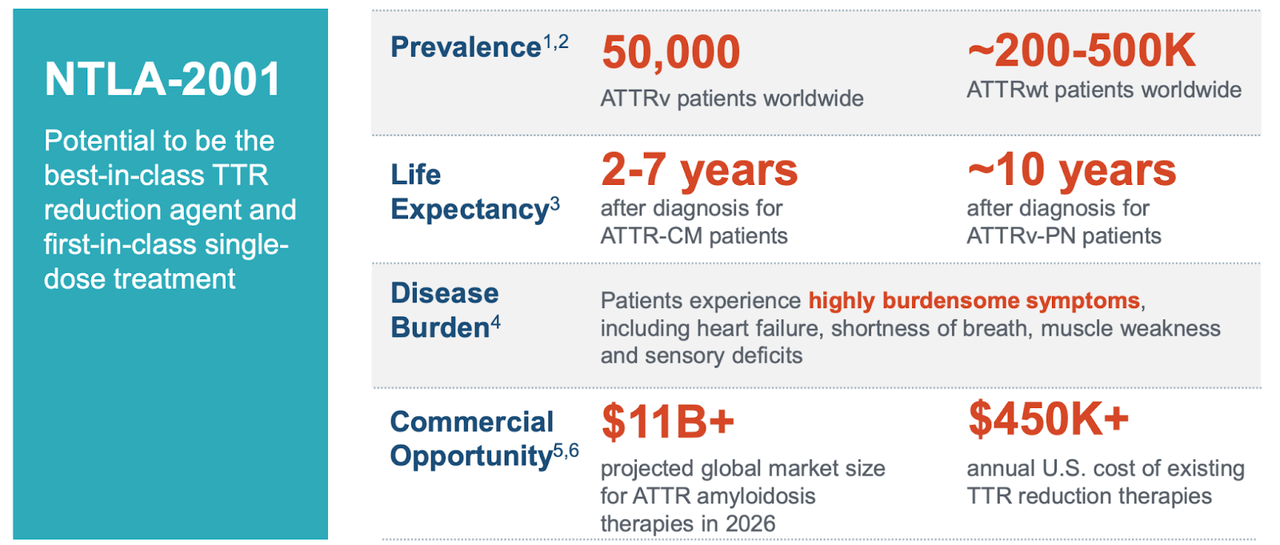
Intellia Therapeutics Progressing Pipeline Signals Positive Outlook Ntla 2002 for hae: initiate phase 2 portion of the ongoing ntla 2002 phase 1 2 study in 1h 2023. submit an ind in 1h 2023 to support inclusion of u.s. sites in the phase 2 study of ntla 2002. present additional clinical data from the ongoing first in human study of ntla 2002 in 2023. alpha 1 antitrypsin deficiency (aatd) franchise:. Conclusions and next steps for ntla 5001. proprietary process enables efficient, scalable genome editing. ~99% ko efficiency of target genes; 50 70% in locus insertion of tgtcrs. sequential editing with high viability and potential for safer products. faster t cell expansion with favorable t cell memory phenotype leading to potentially reduced. First ever clinical data demonstrating redosing with an investigational in vivo crispr based therapyfollow on dosing with a 55 mg dose of ntla 2001 led to a 90% median reduction in serum ttr at. Cambridge, mass., march 18, 2024 (globe newswire) intellia therapeutics, inc. (nasdaq:ntla), a leading clinical stage gene editing company focused on revolutionizing medicine with crispr based.

Comments are closed.