Investigator Role And Responsibilities Ppt
Responsibilities Of Investigator Ppt Video Online Download Investigator role and responsibilities. feb 15, 2020 •. 16 likes • 3,525 views. hsk college of pharmacy. follow. investigator: a person responsible for the conduct of the study at the trial site. investigator is a person responsible for the rights, health and welfare of the study subjects. read more. 1 of 36. Roles and responsibilities of investigator [663] the document discusses the roles and responsibilities of investigators in clinical trials. it states that the principal investigator is responsible for leading the clinical trial team at a trial site and ensuring proper conduct of the trial. key responsibilities include being qualified to conduct.

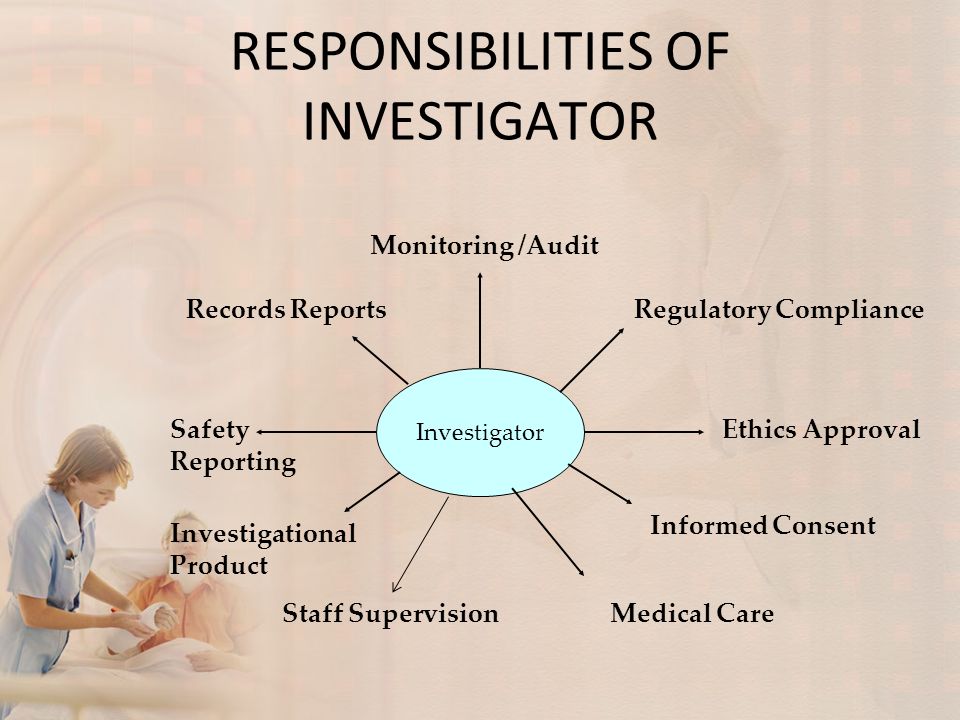
Investigator Role And Responsibilities Ppt An investigator is responsible for leading the clinical trial team and ensuring compliance with regulations. key responsibilities include obtaining necessary approvals, ensuring safety of trial subjects, obtaining informed consent, accurately collecting and reporting data, and protecting subjects' rights and well being. Investigator responsibilities…. •design and implement ethical research, consistent with three ethical principles delineated in the belmont report. •comply with all applicable federal regulations impacting the protection of human subjects. •ensure that all research involving human subjects is submitted to and approved by the appropriate. Investigator responsibilities in clinical research. melissa adde director, clinical trials office, inctr. inctr.org. topics to be discussed. overview of clinical trials good clinical practice the “research team” the role and responsibilities of the principal investigator. 5 sponsor’s responsibilities. quality assurance & quality control to ensure that trials are conducted & data are generated, documented & reported in compliance with the protocol, gcp & applicable regulatory requirements. to ensure direct access to trial related data by the sponsor or inspector (auditor).

Comments are closed.