Lewis Theory Of Bonding Chemwiki
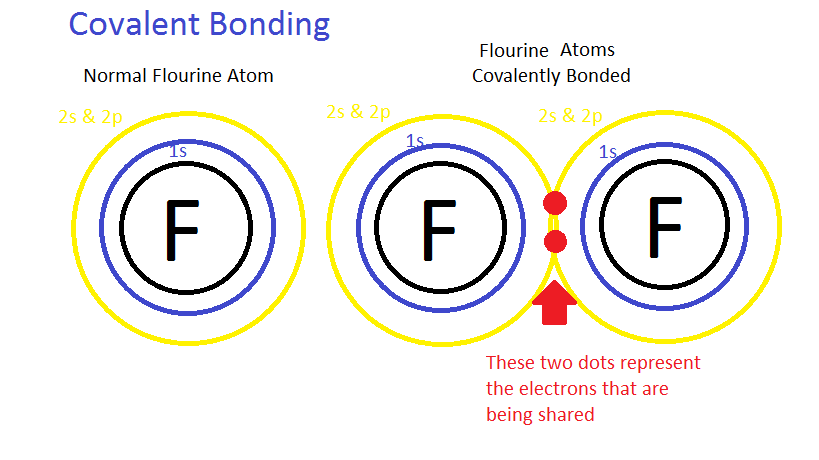
Lewis Theory Of Bonding Chemwiki The lewis theory of bonding essentially combined observations at the time about chemical bonding together. not only was it essential in understanding how elements bonded, it provided a visual representation for them. these lewis dot structures are a simplistic way of representing the electrons in molecules. Lewis theory of bonding is shared under a cc by nc sa 4.0 license and was authored, remixed, and or curated by libretexts. back to top valence shell electron pair repulsion models.

Lewis Theory Of Bonding Chemwiki Lewis used the unpaired dots to predict the number of bonds that an element will form in a compound. consider the symbol for nitrogen in figure 10.1.2 10.1. 2. the lewis dot symbol explains why nitrogen, with three unpaired valence electrons, tends to form compounds in which it shares the unpaired electrons to form three bonds. A lewis acid (named for the american physical chemist gilbert n. lewis) is a chemical species that contains an empty orbital which is capable of accepting an electron pair from a lewis base to form a lewis adduct. a lewis base, then, is any species that has a filled orbital containing an electron pair which is not involved in bonding but may. Lewis acid. lewis theory, generalization concerning acids and bases introduced in 1923 by the u.s. chemist gilbert n. lewis, in which an acid is regarded as any compound which, in a chemical reaction, is able to attach itself to an unshared pair of electrons in another molecule. the molecule with an available electron pair is called a base. Lewis structures – also called lewis dot formulas, lewis dot structures, electron dot structures, or lewis electron dot structures (leds) – are diagrams that show the bonding between atoms of a molecule, as well as the lone pairs of electrons that may exist in the molecule. [1][2][3] a lewis structure can be drawn for any covalently bonded.

Lewis Theory Of Bonding Chemwiki Lewis acid. lewis theory, generalization concerning acids and bases introduced in 1923 by the u.s. chemist gilbert n. lewis, in which an acid is regarded as any compound which, in a chemical reaction, is able to attach itself to an unshared pair of electrons in another molecule. the molecule with an available electron pair is called a base. Lewis structures – also called lewis dot formulas, lewis dot structures, electron dot structures, or lewis electron dot structures (leds) – are diagrams that show the bonding between atoms of a molecule, as well as the lone pairs of electrons that may exist in the molecule. [1][2][3] a lewis structure can be drawn for any covalently bonded. Lewis theory of chemical bonding. in 1916, kossel and lewis explained the reason for noble gases not to combine while other atoms stay in a combined state. they imagined an atom to be like a walnut. as the walnut has a shell (outer shell or valence shell) and kernel (the nucleus and inner electrons), in the same way, they imagined the atom to. In 1923, g.n. lewis from uc berkeley proposed an alternate theory to describe acids and bases. his theory gave a generalized explanation of acids and bases based on structure and bonding. through the use of the lewis definition of acids and bases, chemists are now able to predict a wider variety of acid base reactions.

Lewis Theory Of Bonding Chemwiki Lewis theory of chemical bonding. in 1916, kossel and lewis explained the reason for noble gases not to combine while other atoms stay in a combined state. they imagined an atom to be like a walnut. as the walnut has a shell (outer shell or valence shell) and kernel (the nucleus and inner electrons), in the same way, they imagined the atom to. In 1923, g.n. lewis from uc berkeley proposed an alternate theory to describe acids and bases. his theory gave a generalized explanation of acids and bases based on structure and bonding. through the use of the lewis definition of acids and bases, chemists are now able to predict a wider variety of acid base reactions.

Page Not Found Chemwiki

Comments are closed.