M9q5 6 Molecular Orbital Theory вђ Chem 103 104 Resource Book
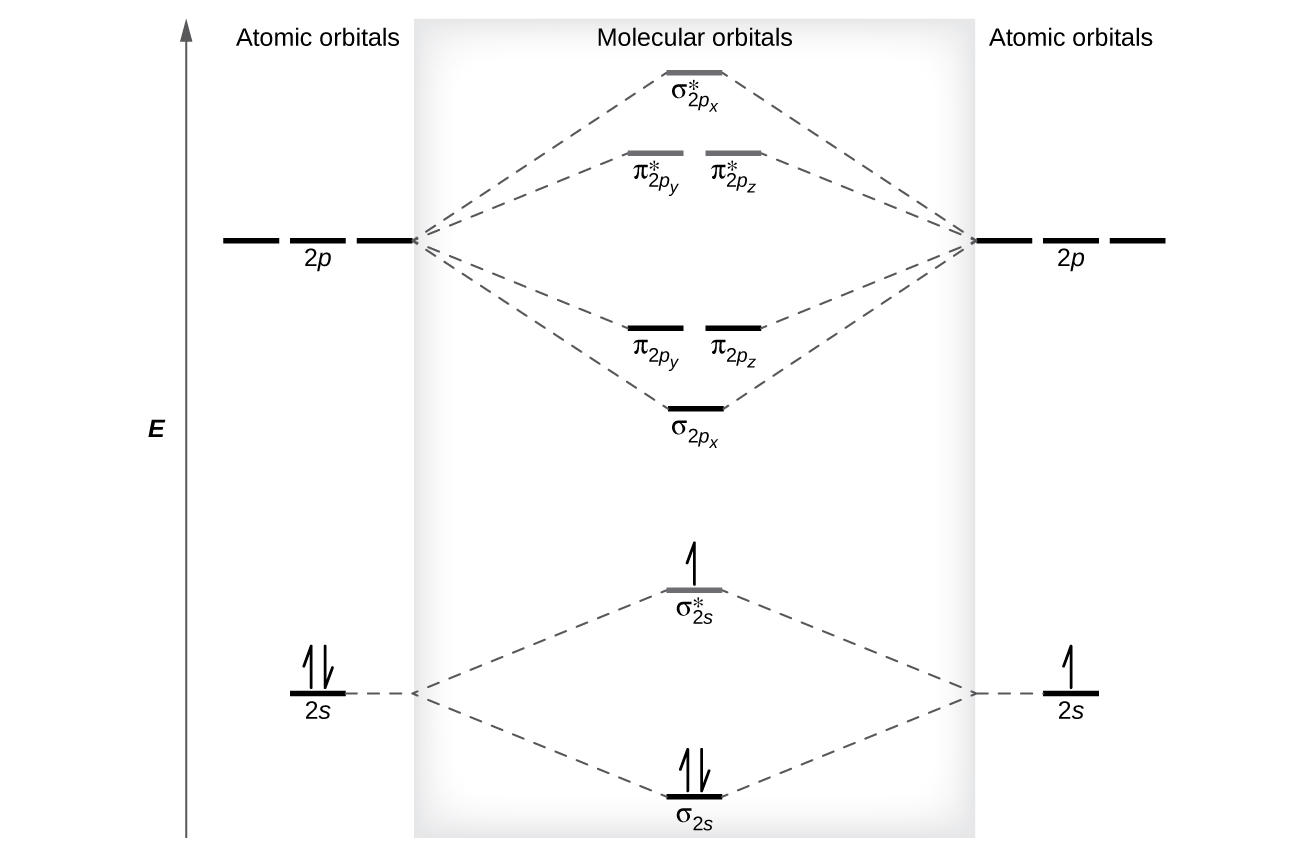
Molecular Orbital Theory M9q5 6 вђ Uw Madison Chemistry 103ођ Molecular orbital theory. considers bonds as localized between one pair of atoms. considers electrons delocalized throughout the entire molecule. creates bonds from overlap of atomic orbitals (s, p, d …) and hybrid orbitals (sp, sp2, sp3 …) combines atomic orbitals to form molecular orbitals (σ, σ*, π, π*) forms σ or π bonds. Molecular orbital theory. considers bonds as localized between one pair of atoms. considers electrons delocalized throughout the entire molecule. creates bonds from overlap of atomic orbitals (s, p, d …) and hybrid orbitals (sp, sp2, sp3 …) combines atomic orbitals to form molecular orbitals (σ, σ*, π, π*) forms σ or π bonds.
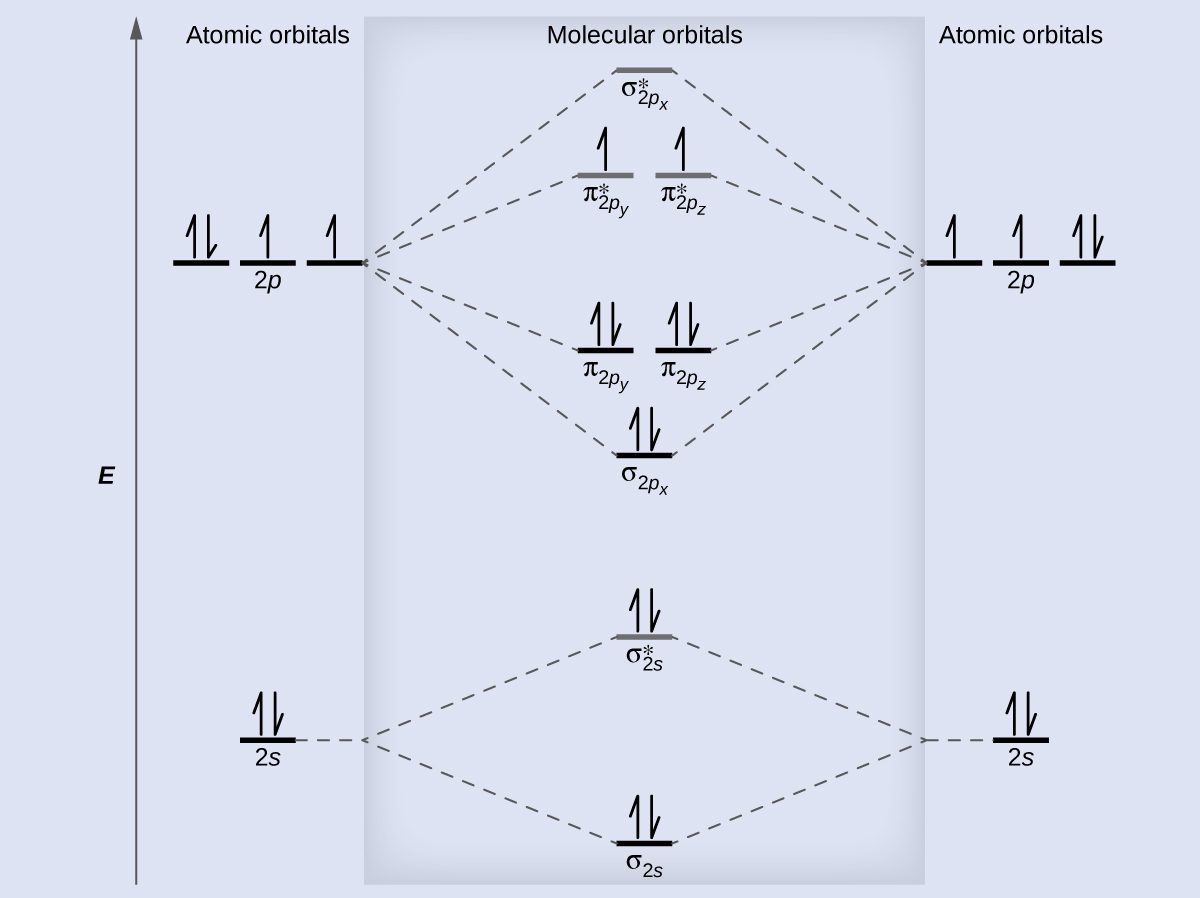
M9q5 6 Molecular Orbital Theory вђ Chem 103 104 R Book title: chem 103 104 resource book m9q5 6: molecular orbital theory. module 10: intermolecular forces. m10q1: an introduction to intermolecular forces. Molecular orbital theory (m9q5 6) x. module 10: intermolecular forces. 53. uw madison chemistry 103 104 resource book. author crlandis license. I am seeking textbook recommendations on molecular orbital theory with comprehensive sections and explanations on diatomic molecules. i want to learn how to construct the molecular orbital energy level diagrams and to write the electronic configuration for diatomic molecules. i have seen several books and papers such as mulliken's papers. Furthermore, vsepr does not provide an explanation of chemical bonding. valence bond theory describes a covalent bond as the overlap of singly occupied atomic orbitals that yield a pair of electrons shared between the two bonded atoms. we say that orbitals on two different atoms overlap when a portion of one orbital and a portion of a second.

Comments are closed.