Main Kinds Of Chemical Reactions

Types Of Chemical Reactions The four main types of chemical reactions are synthesis, decomposition, single displacement, and double displacement. but, remember, some people use different names for these reactions. other important types of reactions are combustion, acid base, redox reactions, and condensation reactions. it gets even more complicated in organic chemistry. The main types of chemical reactions . there are hundreds or even thousands of types of chemical reactions! if you are asked to name the main 4, 5 or 6 types of chemical reactions, here is how they are categorized. the main four types of reactions are direct combination, analysis reaction, single displacement, and double displacement.
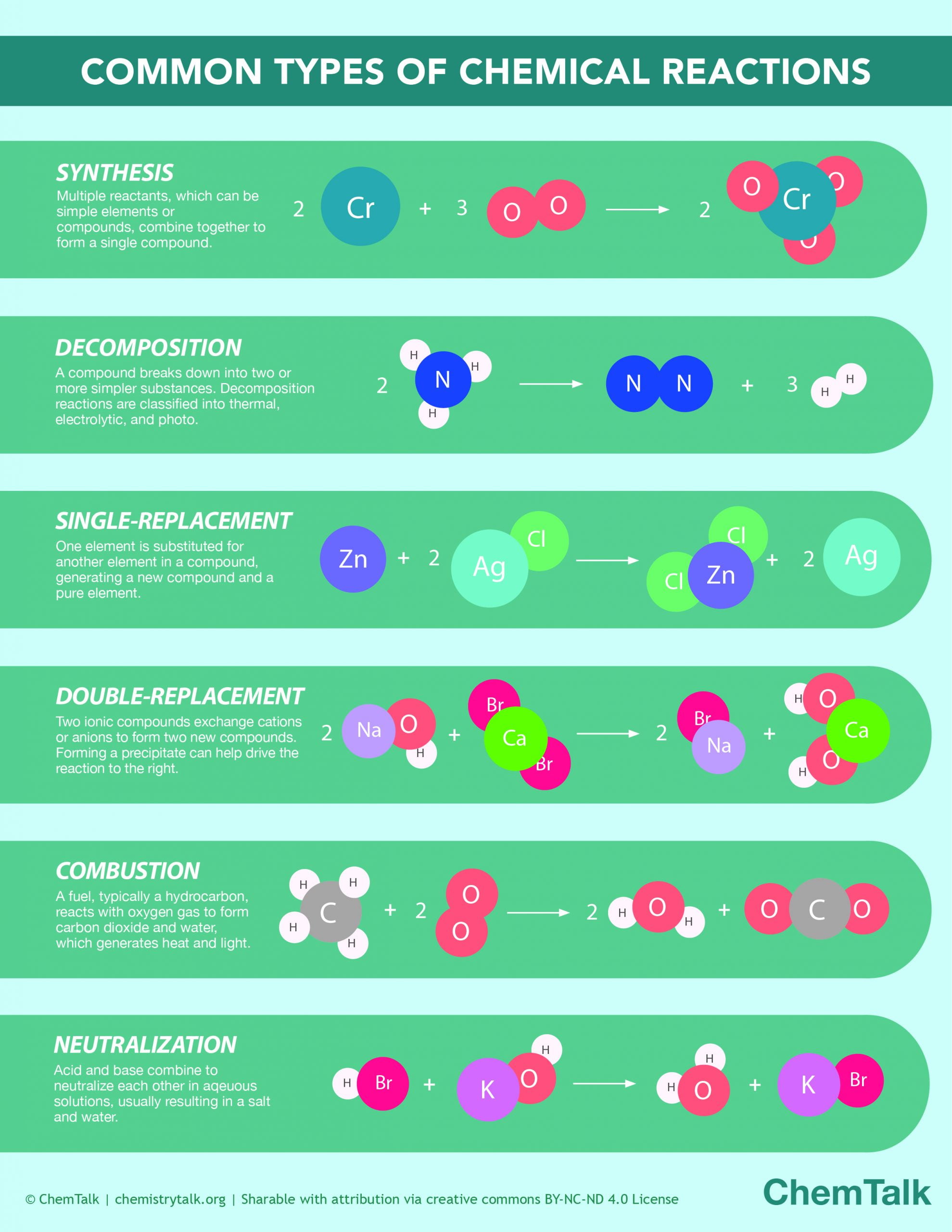
Chemical Reactions Infographic Chemtalk The 5 primary types of chemical reactions are: combination reaction. decomposition reaction. displacement reaction. double displacement reaction. precipitation reaction. 1. combination reaction. a reaction in which two or more reactants combine to form a single product is known as a combination reaction. Many chemical reactions can be classified as one of five basic types. having a thorough understanding of these types of reactions will be useful for predicting the products of an unknown reaction. the five basic types of chemical reactions are combination, decomposition, single replacement, double replacement, and combustion. A chemical reaction is a chemical change, which means the starting materials are chemically different from the ending materials. in contrast, matter also changes form via physical changes. but, in a physical change, the chemical identity of matter does not change. for example, when you melt an ice cube into liquid water, the chemical identity. Summary. chemical reactions may be classified as an a cid–base reaction, an exchange reaction, a condensation reaction and its reverse, a cleavage reaction, and an oxidation–reduction (or redox) reaction. to keep track of electrons in chemical reactions, oxidation states are assigned to atoms in compounds.

Types Of Chemical Reactions A chemical reaction is a chemical change, which means the starting materials are chemically different from the ending materials. in contrast, matter also changes form via physical changes. but, in a physical change, the chemical identity of matter does not change. for example, when you melt an ice cube into liquid water, the chemical identity. Summary. chemical reactions may be classified as an a cid–base reaction, an exchange reaction, a condensation reaction and its reverse, a cleavage reaction, and an oxidation–reduction (or redox) reaction. to keep track of electrons in chemical reactions, oxidation states are assigned to atoms in compounds. Types of chemical reactions : core concepts. this article will cover the main classifications of chemical reactions: synthesis reaction, decomposition reaction, single replacement reaction (single displacement reaction), and double replacement reaction (double displacement reaction). we also discuss what is a combustion reaction, precipitation. The state of matter of reactants and products is designated with the symbols (s) for solids, (l) for liquids, and (g) for gases. chemical reaction, a process in which one or more substances, the reactants, are converted to one or more different substances, the products. substances are either chemical elements or compounds.

Types Of Chemical Reactions Detailed Explanation With Example Videos Types of chemical reactions : core concepts. this article will cover the main classifications of chemical reactions: synthesis reaction, decomposition reaction, single replacement reaction (single displacement reaction), and double replacement reaction (double displacement reaction). we also discuss what is a combustion reaction, precipitation. The state of matter of reactants and products is designated with the symbols (s) for solids, (l) for liquids, and (g) for gases. chemical reaction, a process in which one or more substances, the reactants, are converted to one or more different substances, the products. substances are either chemical elements or compounds.

Comments are closed.