Manual Print Serialization System For Pharmaceuticals

Manual Print Station Mps Serialization Product Solution Optel Pce’s manual serialization station (mss) provides all the components required for serialization operations in a compact and portable desktop machine. featuring an adjustable inkjet print unit with integrated print inspection capabilities, the mss allows for a variety of carton sizes, as well as a bluetooth hand scanner for code verification. The manual print station (mps) is an ultra compact and fully autonomous serialization and aggregation solution. it efficiently prints serialization identific.

Serialization System In Pharma Products By integrating with the tracelink network, manufacturers can effortlessly connect with partners in various formats, bypassing the costly and time consuming traditional point to point integrations. our suite of serialization solutions, including snm, snx, and som, marks a new era in pharmaceutical manufacturing, where efficiency meets security. The importance of serialization in pharma manufacturing. serialization is a critical tool used by regulatory agencies to prove the validity of pharmaceutical products, enabling them to track the item (s) back to the original manufacturer. the serialization process can also provide a lot of value to the manufacturer as well. The most important requirement for any pharma company implementing serialization is to minimize interruption to your business operation. optel has 3 defined stages of implementation: phase 2: planning. phase 3: validation and support. phase 1: thorough analysis. phase 2: planning. phase 3: validation and support. phase 1: thorough analysis. Gned into law, with full implementation phased in over the next 10 years. the next milestone for manufacturers, november 27, 2017, is the date by which pharmaceutical manufacturers are required to print a unique product identification. cases distributed domestically.serialization: delay no longer an optionstarting november 27, 2017.
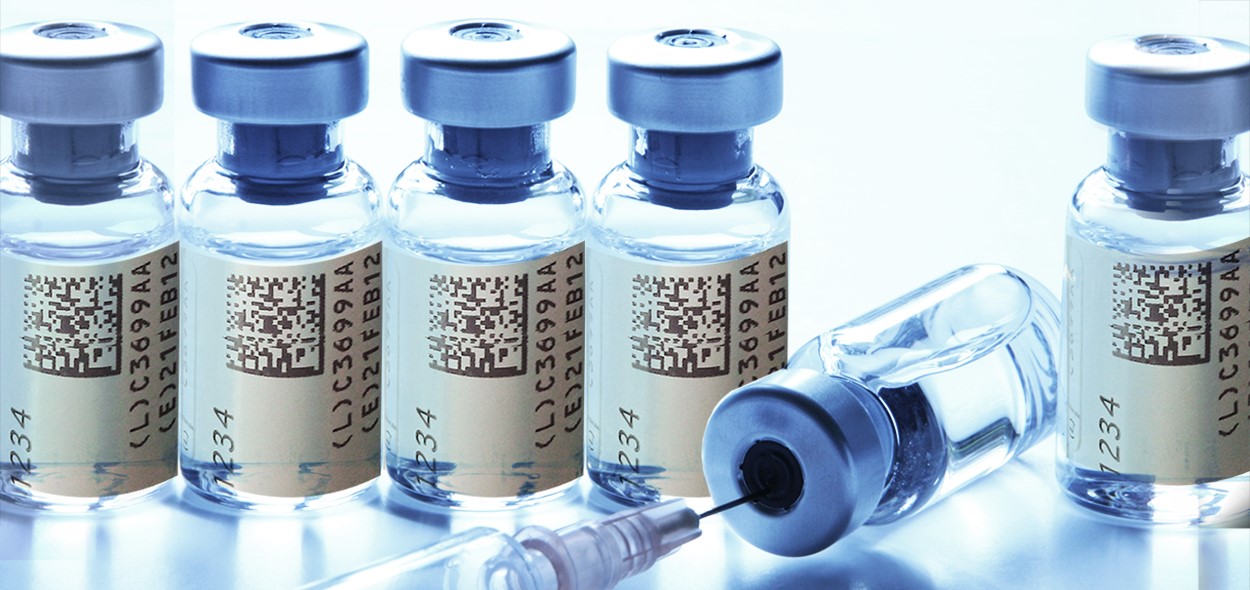
Pharma Product Serialization The most important requirement for any pharma company implementing serialization is to minimize interruption to your business operation. optel has 3 defined stages of implementation: phase 2: planning. phase 3: validation and support. phase 1: thorough analysis. phase 2: planning. phase 3: validation and support. phase 1: thorough analysis. Gned into law, with full implementation phased in over the next 10 years. the next milestone for manufacturers, november 27, 2017, is the date by which pharmaceutical manufacturers are required to print a unique product identification. cases distributed domestically.serialization: delay no longer an optionstarting november 27, 2017. Pharmaceutical serialization is a pivotal strategy in the global fight against counterfeit drugs, ensuring the authenticity and safety of pharmaceutical products throughout their journey from production to distribution. this article delves into the essential aspects of pharmaceutical serialization, exploring its definition and significance in today's regulatory landscape. it outlines the core. The serialization solution provides an end to end supply chain platform, and offers an aggregation workflow to help customers using manual aggregation processes. new tool: prosource check out our packaging and processing solutions finder, prosource.

Videojet Imprints Ai21邃 Farmaceutisk Serialiseringsplattform Pharmaceutical serialization is a pivotal strategy in the global fight against counterfeit drugs, ensuring the authenticity and safety of pharmaceutical products throughout their journey from production to distribution. this article delves into the essential aspects of pharmaceutical serialization, exploring its definition and significance in today's regulatory landscape. it outlines the core. The serialization solution provides an end to end supply chain platform, and offers an aggregation workflow to help customers using manual aggregation processes. new tool: prosource check out our packaging and processing solutions finder, prosource.

Madras Security Printers

Comments are closed.