Mendeleev Periodic Table Elements Elcho Table
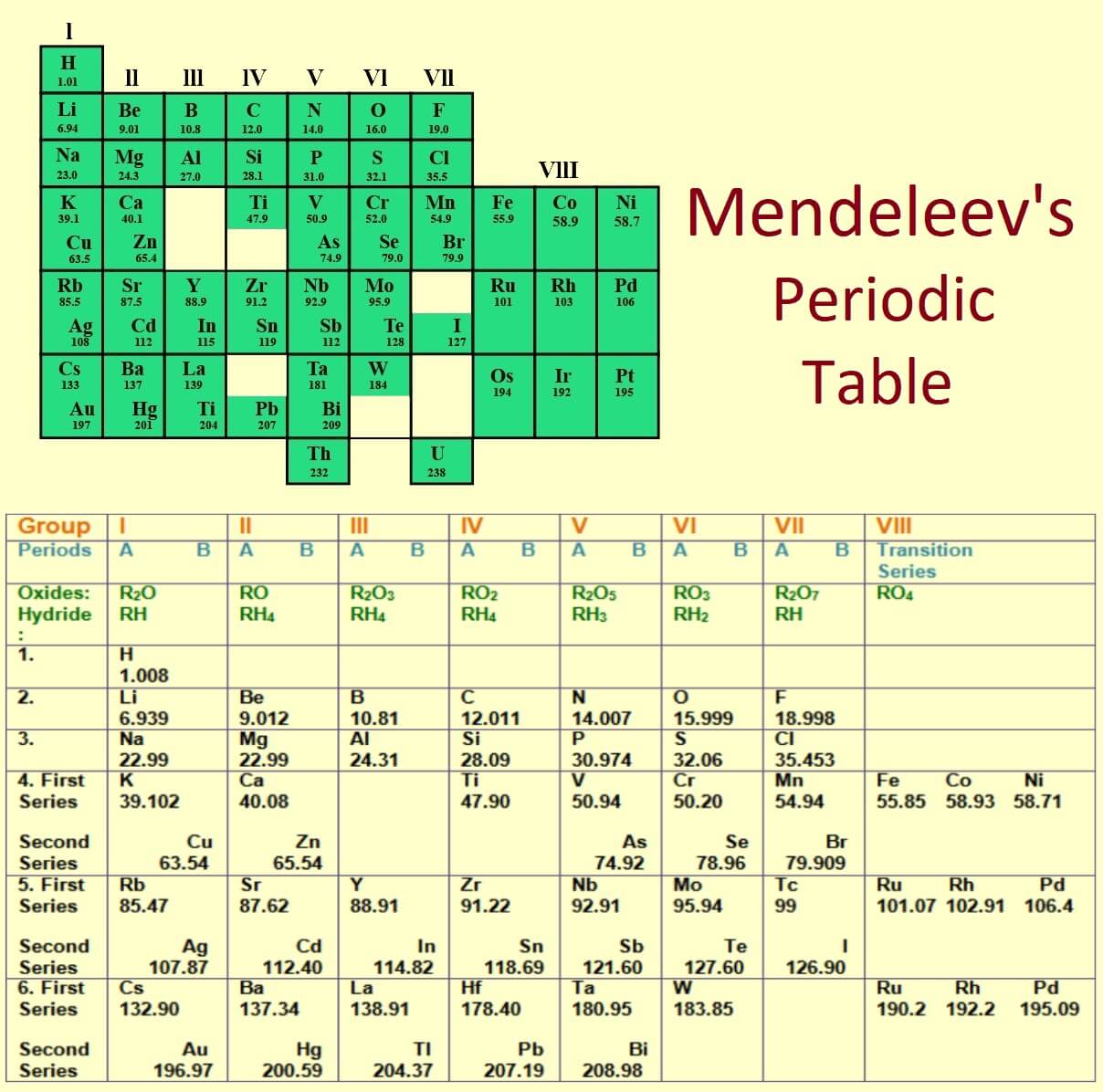
Mendeleev Periodic Table Elements Elcho Table Mendeleev’s first periodic table in 1869 included the 63 known elements and spaces for three predicted, undiscovered elements. he revised and refined this table multiple times, as new data came to light. dmitri mendeleev did not invent the first periodic table. instead, he devised a table that organizes elements by atomic weight and periodic. Mendeleev’s periodic table presented a new paradigm, with all of the elements positioned within a logical matrix. the elements are arranged in a series of rows called “periods,” so that those with similar properties appear in vertical columns. each vertical column is called a “group,” or family, of elements.

Mendeleev Periodic Table Elements Elcho Table My Xxx Hot Girl By adding additional elements following this pattern, mendeleev developed his extended version of the periodic table. [ 35 ] [ 36 ] on 6 march 1869, he made a formal presentation to the russian chemical society, titled the dependence between the properties of the atomic weights of the elements , which described elements according to both atomic. By convention, elements are organized in the periodic table, a structure that captures important patterns in their behavior.devised by russian chemist dmitri mendeleev (1834–1907) in 1869, the table places elements into columns—groups—and rows—periods—that share certain properties. Mendeleev’s legacy: the periodic system. mendeleev’s greatest achievement was not the periodic table so much as the recognition of the periodic system on which it was based. this year marks the 100th anniversary of the death of one of the most famous scientists of all time, the russian chemist dmitri ivanovich mendeleev (1834–1907). Firstly, mendeleev's table was based on atomic mass, while the modern table is based on atomic number. this change was crucial in accurately organizing elements and predicting their properties. secondly, the modern periodic table has a more precise organization of elements into periods and groups, allowing for a better understanding of trends.
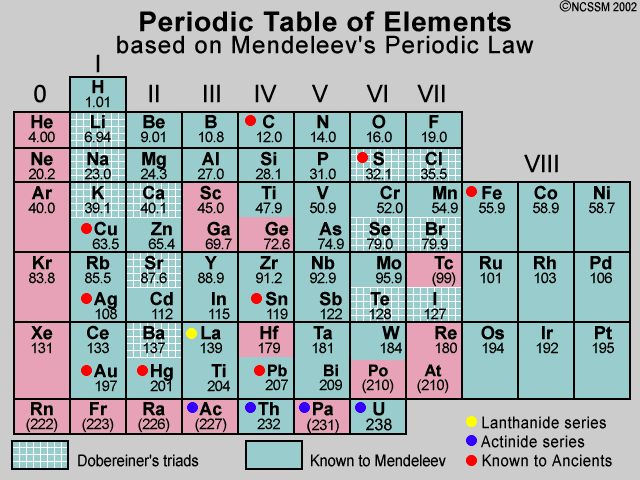
2 Mendeleev S Periodic Table Notes Ncert Solutions For Cbse Class 10 Mendeleev’s legacy: the periodic system. mendeleev’s greatest achievement was not the periodic table so much as the recognition of the periodic system on which it was based. this year marks the 100th anniversary of the death of one of the most famous scientists of all time, the russian chemist dmitri ivanovich mendeleev (1834–1907). Firstly, mendeleev's table was based on atomic mass, while the modern table is based on atomic number. this change was crucial in accurately organizing elements and predicting their properties. secondly, the modern periodic table has a more precise organization of elements into periods and groups, allowing for a better understanding of trends. Dmitri mendeleev (born january 27 (february 8, new style), 1834, tobolsk, siberia, russian empire—died january 20 (february 2), 1907, st. petersburg, russia) was a russian chemist who developed the periodic classification of the elements. mendeleev found that, when all the known chemical elements were arranged in order of increasing atomic. Listed in the table below are other properties that mendeleev predicted for the first of these two missing elements, which he called "eka aluminum", compared with the element gallium. mendeleev's predicted properties for eka aluminum and gallium. eka aluminum (ea) ( ea) gallium (ga) ( ga) atomic mass. 68amu 68 amu. 69.9amu 69.9 amu.

Comments are closed.