Mendeleev S Periodic Table
Chemical Properties And Usage Ssc Chemistry Russian chemist and educator dmitrii mendeleev is best known today for his creation of the periodic table of elements. mendeleev was far from the first chemist to attempt to organize the elements by atomic weight or to recognize that characteristics recurred on some sort of regular basis. Key points. mendeleev’s first periodic table in 1869 included the 63 known elements and spaces for three predicted, undiscovered elements. he revised and refined this table multiple times, as new data came to light. dmitri mendeleev did not invent the first periodic table.
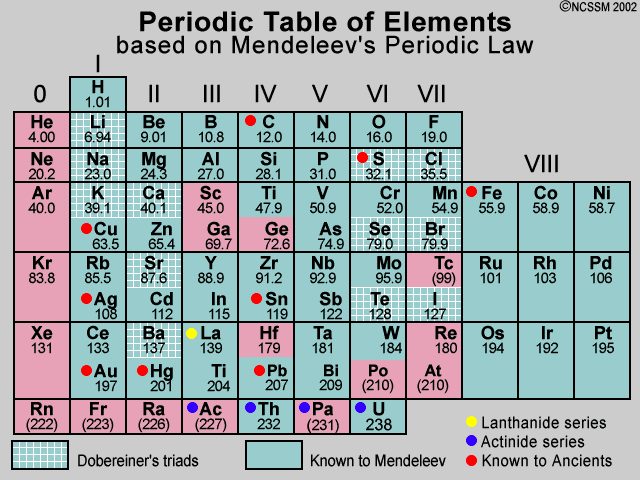
Mendeleev S Periodic Table Dmitri mendeleev, a russian chemist and teacher, devised the periodic table — a comprehensive system for classifying the chemical elements. organizing matter. in the mid 1700s, chemists began actively identifying elements, which are substances made up of just one kind of atom. Dmitri mendeleev (born january 27 (february 8, new style), 1834, tobolsk, siberia, russian empire—died january 20 (february 2), 1907, st. petersburg, russia) was a russian chemist who developed the periodic classification of the elements. Dmitri ivanovich mendeleev formemrs (sometimes romanized as mendeleyev, mendeleiev, or mendeleef; english: ˌmɛndəlˈeɪəf men dəl ay əf; [ 2] russian: Дмитрий Иванович Менделеев, romanized: dmitriy ivanovich mendeleyev, [ a] ipa: [ˈdmʲitrʲɪj ɪˈvanəvʲɪtɕ mʲɪnʲdʲɪˈlʲejɪf] ⓘ; 8 february [ o.s. 27 january] 1834 – 2 february [o.s. 20 january] 1907). Dmitri ivanovich mendeléev, a russian chemist, was the most important contributor to the early development of the periodic table. many periodic tables were made but the most important one was the mendeleev periodic table. in 1869, after the rejection of newlands octave law, mendeleev periodic table came into the picture.

Mendeleev S Periodic Table Class 10 Periodic Classification Of Elements Dmitri ivanovich mendeleev formemrs (sometimes romanized as mendeleyev, mendeleiev, or mendeleef; english: ˌmɛndəlˈeɪəf men dəl ay əf; [ 2] russian: Дмитрий Иванович Менделеев, romanized: dmitriy ivanovich mendeleyev, [ a] ipa: [ˈdmʲitrʲɪj ɪˈvanəvʲɪtɕ mʲɪnʲdʲɪˈlʲejɪf] ⓘ; 8 february [ o.s. 27 january] 1834 – 2 february [o.s. 20 january] 1907). Dmitri ivanovich mendeléev, a russian chemist, was the most important contributor to the early development of the periodic table. many periodic tables were made but the most important one was the mendeleev periodic table. in 1869, after the rejection of newlands octave law, mendeleev periodic table came into the picture. Mendeleev’s legacy: the periodic system. mendeleev’s greatest achievement was not the periodic table so much as the recognition of the periodic system on which it was based. byeric scerri. print republish google classroom. about support our work. Mendeleev ’s periodic table of 1869 contained 17 columns, with two nearly complete periods (sequences) of elements, from potassium to bromine and rubidium to iodine, preceded by two partial periods of seven elements each (lithium to fluorine and sodium to chlorine), and followed by three incomplete periods.
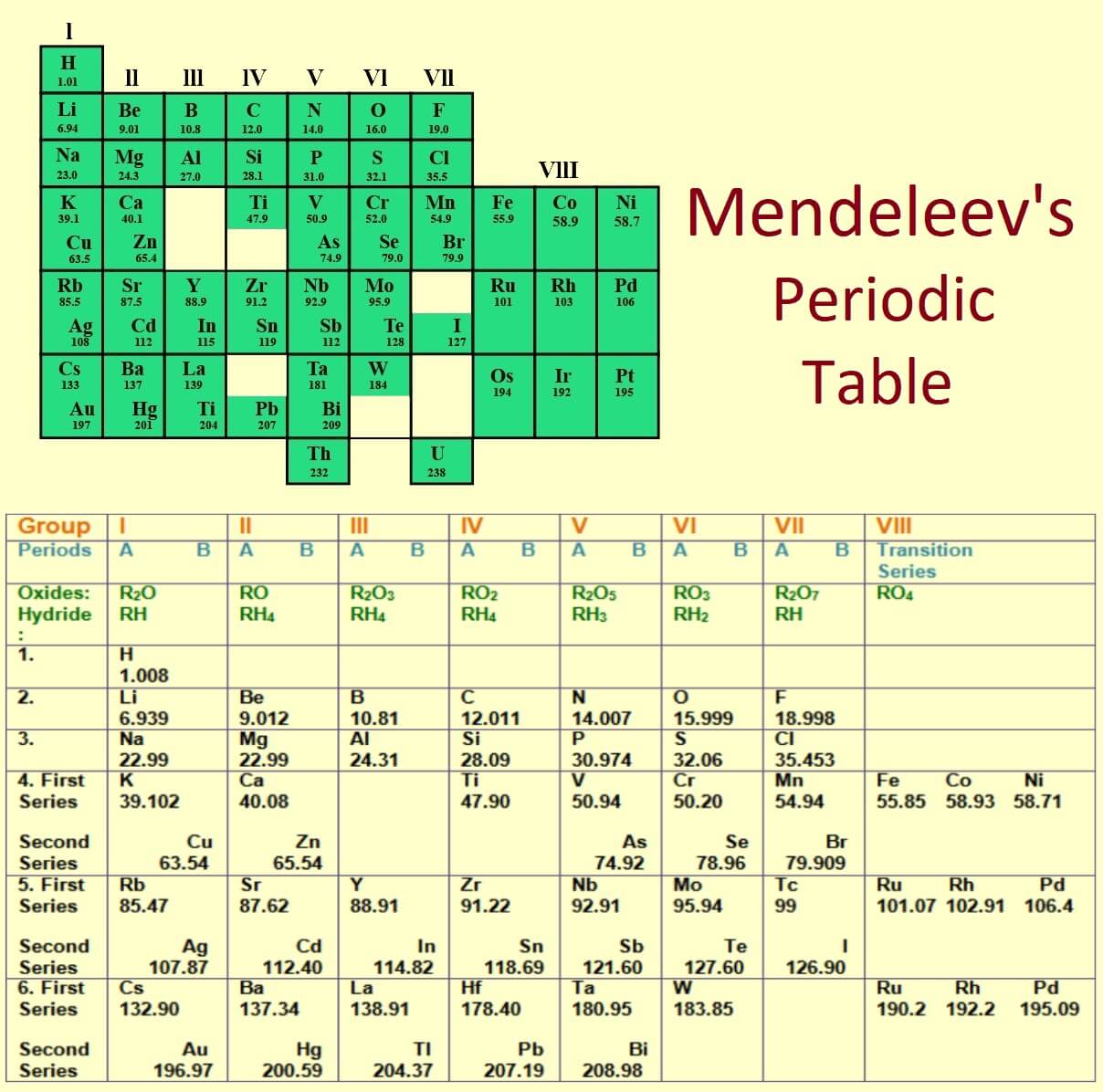
Periodic Table Dmitri Mendeleev Mendeleev S Periodic Table Class The Mendeleev’s legacy: the periodic system. mendeleev’s greatest achievement was not the periodic table so much as the recognition of the periodic system on which it was based. byeric scerri. print republish google classroom. about support our work. Mendeleev ’s periodic table of 1869 contained 17 columns, with two nearly complete periods (sequences) of elements, from potassium to bromine and rubidium to iodine, preceded by two partial periods of seven elements each (lithium to fluorine and sodium to chlorine), and followed by three incomplete periods.

юааmendeleevтащs Periodic Tableюаб Arrangements Importance Limitations Faq

Comments are closed.