Molecular Orbital Mo Diagram For O2

O2 Molecular Orbital Diagrams 101 Diagrams Steps for drawing the molecular orbital (mo) diagram of o 2 with its bond order. 1. write down the electronic configuration of o 2 atoms . o 2 consists of two oxygen (o) atoms the electronic configuration of each o atom is 1s 2 2s 2 2p x 1 2p y 1 2p z 2. Solution. the bond length in the oxygen species can be explained by the positions of the electrons in molecular orbital theory. to obtain the molecular orbital energy level diagram for \(\ce{o2}\), we need to place 12 valence electrons (6 from each o atom) in the energy level diagram shown in figure 9.10.1 .
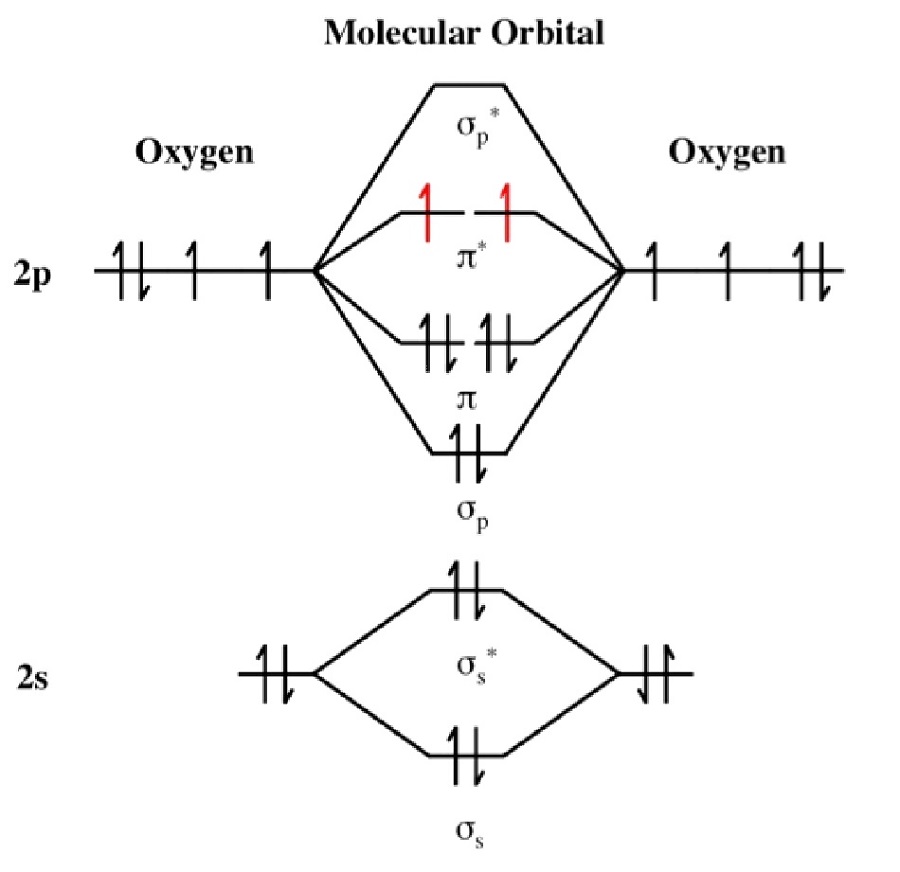
O2 Molecular Orbital Diagrams 101 Diagrams When two oxygen atoms overlap, the sigma(2p) molecular orbital is lower in energy than the pi(2p) orbitals. this different from nitrogen, where it's the othe. Remember: when two oxygen atoms bond, the pi(2p) bonding molecular orbitals are lower in energy than the sigma(2p) bonding orbitals. they are flipped compare. We draw a molecular orbital energy diagram similar to that shown in figure \(\pageindex{7}\). each oxygen atom contributes six electrons, so the diagram appears as shown in figure \(\pageindex{7}\). figure \(\pageindex{13}\): the molecular orbital energy diagram for o 2 predicts two unpaired electrons. we calculate the bond order as. We draw a molecular orbital energy diagram similar to that shown in figure 7.7.12. each oxygen atom contributes six electrons, so the diagram appears as shown in figure 7.7.15. figure 7.7.15. the molecular orbital energy diagram for [latex]\ce{o2}[ latex] predicts two unpaired electrons. we calculate the bond order as.

Comments are closed.