Multistage Clinical Trial Phases With Cost Involved Rules Pdf
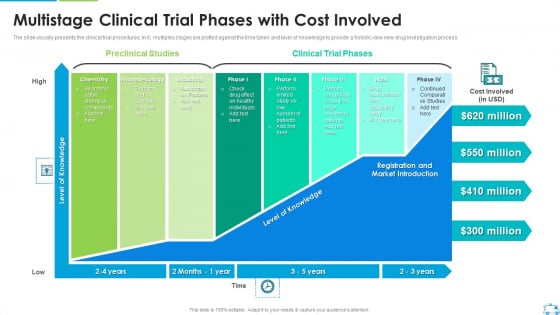
Multistage Clinical Trial Phases With Cost Involved Rules Pdf One such adaptive design is the mams trial. mams trials were first reported over 20 years ago as a way to accelerate the process of drug development.3. mams has been more commonly implemented in phase ii iii settings, although it can be applied in any trial phase. rather than a series of separate phase ii iii studies, mams trials aim to answer. Clinical trials can be separated into phase i (dose finding and safety), phase ii (activity or early efficacy), phase iii (efficacy compared with current standard of care) and occasionally phase iv (postmarketing studies). a new compound would usually have to go through phase i–iii studies sequentially with all of the financial and regulatory hurdles this poses. a recent study has estimated.

Multistage Clinical Trial Powerpoint Templates Slides And Graphics Randomised controlled trials are becoming increasingly costly and time consuming. in 2011, royston and colleagues proposed a particular class of multi arm multi stage (mams) designs intended to speed up the evaluation of new treatments in phase ii and iii clinical trials. This paper proposes a generic bayesian adaptive decision theoretic design for multi arm multi stage clinical trials with k ( k ≥ 2 ) arms and defines a loss function that incorporates the patient accrual costs as well as costs associated with an incorrect decision at the end of the trial. Background in recent years, the popularity of multi arm multi stage, seamless adaptive, and platform trials has increased. however, many design related questions and questions regarding which operating characteristics should be evaluated to determine the potential performance of a specific trial design remain and are often further complicated by the complexity of such trial designs. methods a. Background interest in and use of electronic consent (e consent) in the conduct of academic clinical trials has increased since the covid 19 pandemic. e consent offers advantages including increased efficiency and accessibility, and reduced burden on site staff, which can be appealing to academic trialists anticipating challenges in recruitment to complex trial designs or with limited funding.

Comments are closed.