Multistage Clinical Trial Phases With Licensing New Clinical
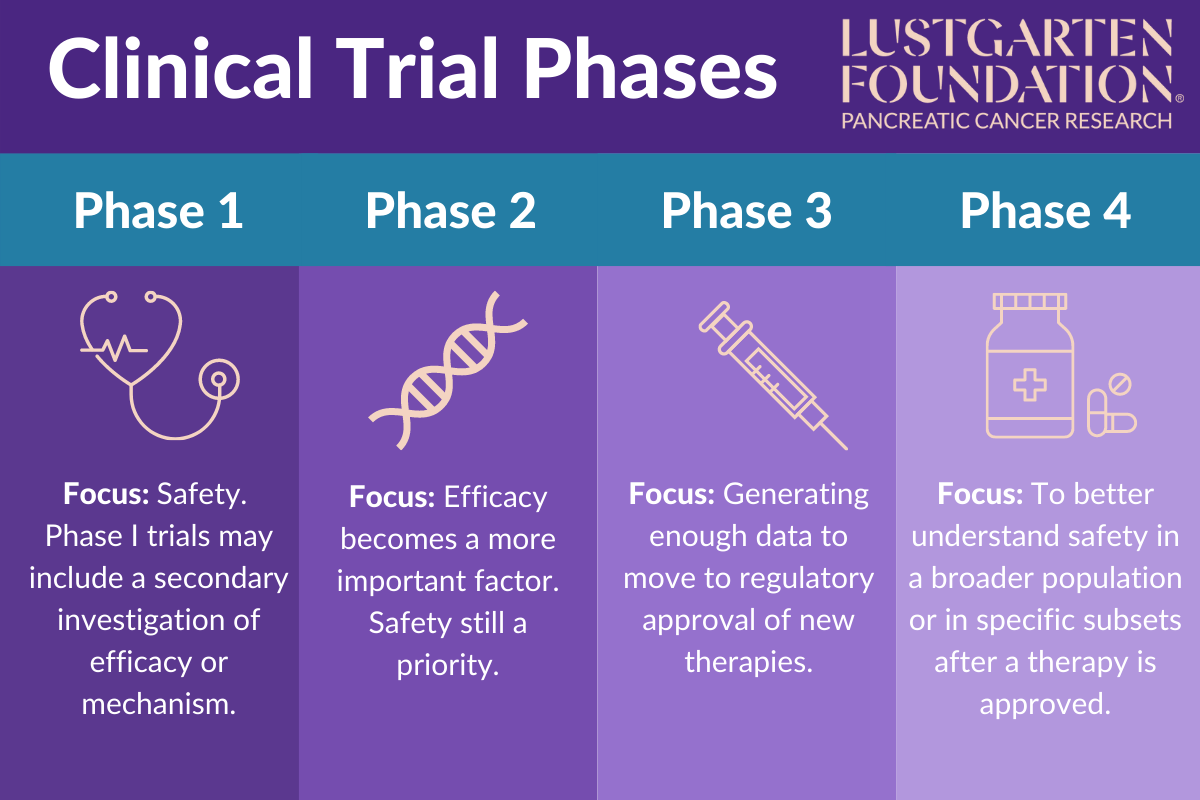
Clinical Trial Phases Clinical trials can be separated into phase i (dose finding and safety), phase ii (activity or early efficacy), phase iii (efficacy compared with current standard of care) and occasionally phase iv (postmarketing studies). a new compound would usually have to go through phase i–iii studies sequentially with all of the financial and regulatory hurdles this poses. a recent study has estimated. One such adaptive design is the mams trial. mams trials were first reported over 20 years ago as a way to accelerate the process of drug development.3. mams has been more commonly implemented in phase ii iii settings, although it can be applied in any trial phase. rather than a series of separate phase ii iii studies, mams trials aim to answer.

Multistage Clinical Trial Phases With Licensing Medical Research Ph We know that for a standard phase iii trial with α = .025 and β = .10, the probability of a false positive is 0.025 and the probability of a false negative is 0.10. with a 2 stage phase iii design, the per study false positive rate is p 0 ∗ ( win) while the per study false negative rate is 1 − p 0 ∗ ( win). power is p 0 ∗ ( win). Increasing uptake of late phase trials using the multi arm multi stage (mams) platform approach over time. hosepipe plot demonstrating increasing uptake of mams platform for late phase clinical trials over time, accelerated by uptake during the covid 19 pandemic. the date of lock for new trial registrations was 1 april 2021. Randomised controlled trials are becoming increasingly costly and time consuming. in 2011, royston and colleagues proposed a particular class of multi arm multi stage (mams) designs intended to speed up the evaluation of new treatments in phase ii and iii clinical trials. New agents in oncology historically do not progress beyond early phase clinical trials, leading to the exorbitant costs and lengthy time courses associated with the drug development process 8; however, a recent study illustrated that well performing dose finding designs can have a tremendous impact on the drug development process. 9.
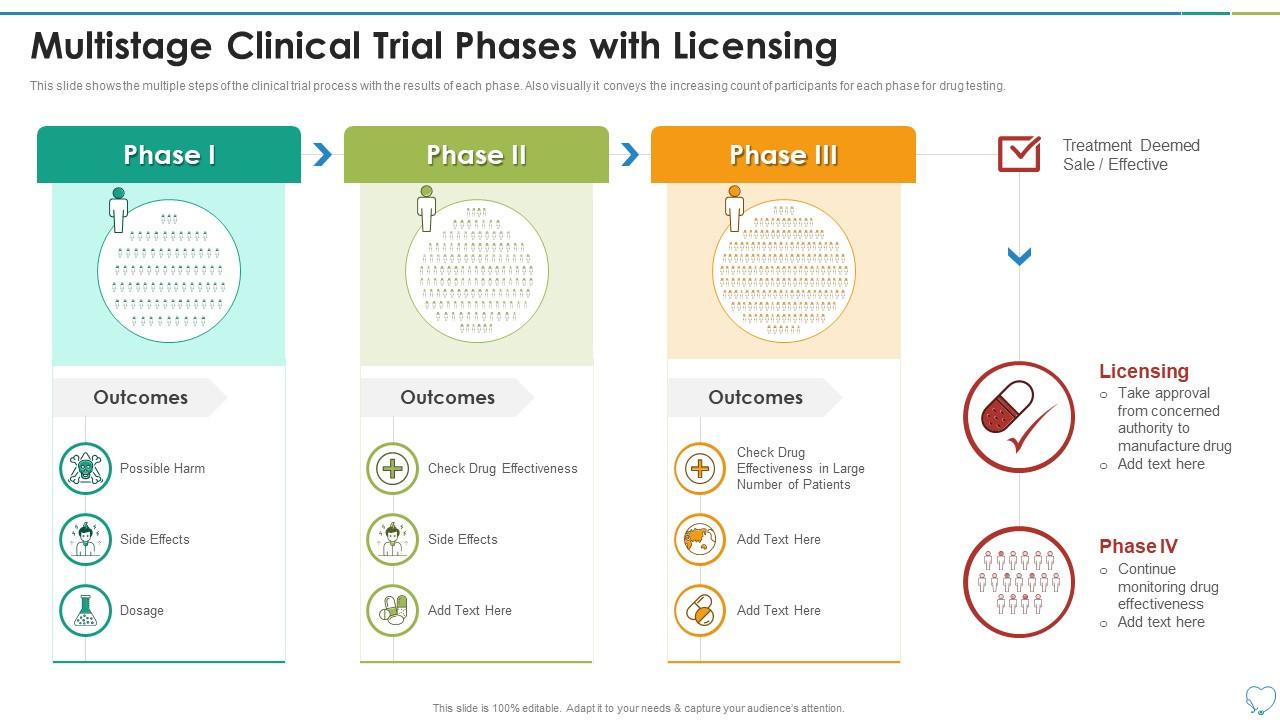
Clinical Trial Phases Multistage Clinical Trial Phases Randomised controlled trials are becoming increasingly costly and time consuming. in 2011, royston and colleagues proposed a particular class of multi arm multi stage (mams) designs intended to speed up the evaluation of new treatments in phase ii and iii clinical trials. New agents in oncology historically do not progress beyond early phase clinical trials, leading to the exorbitant costs and lengthy time courses associated with the drug development process 8; however, a recent study illustrated that well performing dose finding designs can have a tremendous impact on the drug development process. 9. Two methods for designing adaptive multiarm multistage (mams) clinical trials, originating from conceptually different group sequential frameworks are presented, and their operating characteristics are compared. in both methods pairwise comparisons are made, stage by stage, between each treatment arm and a common control arm with the goal of. Clinical trials follow a particular timeline, from early, small scale, phase 1 studies to late stage, large scale, phase 3 studies.1 while there are many steps involved in the development of new drugs, clinical trials, which make up clinical research, are the part of drug development that involves people. here we describe the key goals and.

Comments are closed.