New Drug Development Process Clinical Trial Phases Funnel With Drug
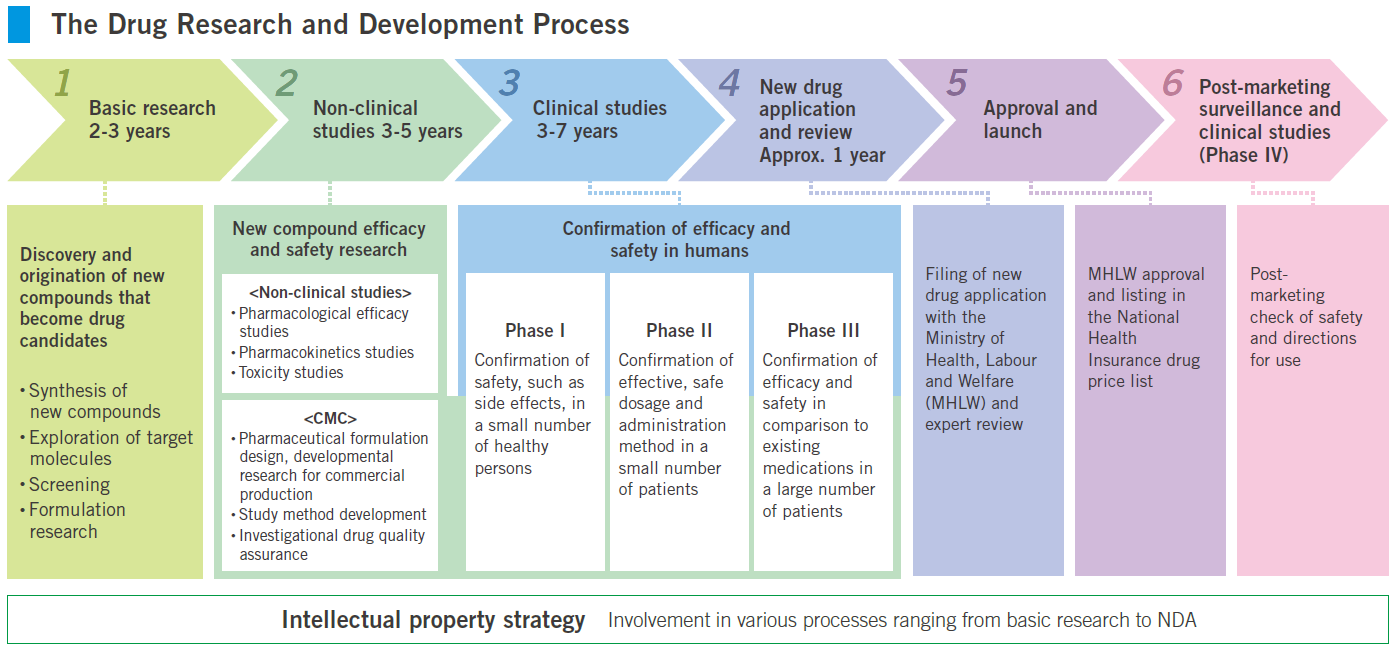
An Overview Of New Drug Discovery And Development Pharmatutor Share. step 1 discovery and development. discovery and development research for a new drug begins in the laboratory. more information. step 2 preclinical research. preclinical research drugs. Drug development funnel . fda review clinical trials “the drug development process is lengthy and detailed, but it is a logical path with clear milestones.” titled “content and.
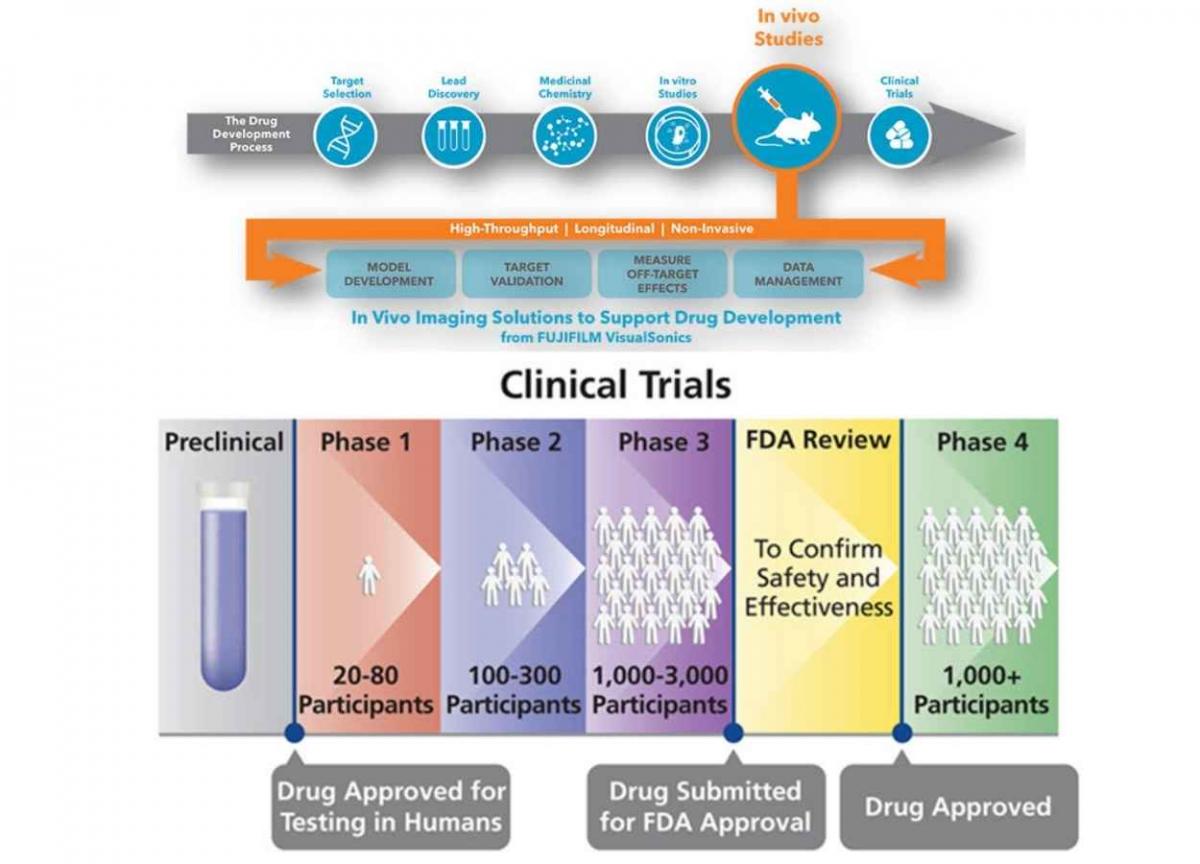
Custom Writing Service Www Fuste Pt Watch this video to learn about the three phases of clinical trials. clinical research phase studies. phase 1. study participants: 20 to 100 healthy volunteers or people with the disease condition. The drug development process is a comprehensive and multiphase journey that transforms scientific discoveries into life saving medicines. from the initial drug discovery phase to clinical trials and regulatory drug approval, each stage is crucial in ensuring the success of new medications. this intricate process requires collaboration. The limited size allows for close monitoring and detailed data collection without exposing too many individuals to potential risks. phase 1 clinical trials are a pivotal step in drug development, laying the groundwork for subsequent phases by providing essential safety and dosage information. phase 2 clinical trials. Average cash estimated per success drug b clinical development time, years (pi pii piii) real annual coc (rate) average capitalized costs estimated per successful drug b; clinical phases total c clinical phases (pi, pii and piii) total c; wouters et al. —all: 2020: mixed: fda approval between 2009 and 2018: 63 nces and nbes: 355 nces and nbes.

Drug Delivery Devices Throughout The Drug Development Cycle The limited size allows for close monitoring and detailed data collection without exposing too many individuals to potential risks. phase 1 clinical trials are a pivotal step in drug development, laying the groundwork for subsequent phases by providing essential safety and dosage information. phase 2 clinical trials. Average cash estimated per success drug b clinical development time, years (pi pii piii) real annual coc (rate) average capitalized costs estimated per successful drug b; clinical phases total c clinical phases (pi, pii and piii) total c; wouters et al. —all: 2020: mixed: fda approval between 2009 and 2018: 63 nces and nbes: 355 nces and nbes. Pharmaceutical company can begin to test the potential new drug in humans. this process includes three phases of clinical trials. phase 1 trials a new drug is administered to approximately 20 to 80 healthy volunteers, to study the activity and monitor potential toxicity in people. this process takes about one year and if. At this stage, the project focus shifts from drug discovery to drug development to enable human clinical trials. if the therapeutic agent is successful in all three phases of the clinical trials, it goes through regulatory registration and the drug can be marketed ( hefti, 2008; hughes et al., 2011; mohs and greig, 2017 ).

Exploring The Drug Development Process Technology Networks Pharmaceutical company can begin to test the potential new drug in humans. this process includes three phases of clinical trials. phase 1 trials a new drug is administered to approximately 20 to 80 healthy volunteers, to study the activity and monitor potential toxicity in people. this process takes about one year and if. At this stage, the project focus shifts from drug discovery to drug development to enable human clinical trials. if the therapeutic agent is successful in all three phases of the clinical trials, it goes through regulatory registration and the drug can be marketed ( hefti, 2008; hughes et al., 2011; mohs and greig, 2017 ).

Comments are closed.