New Treatment Protocol For Previously Untreatable Multiple Myeloma

Jsm 2024 Myeloma Cells Waly Amalita Front line treatment patterns in multiple myeloma: an analysis of u.s. based electronic health records from 2011 to 2019. cancer med 2021;10:5866 5877. crossref. "immunotherapy is the new kid on the block if you will, but it has a huge role in the treatment of multiple myeloma," says dr. lin. now a standard treatment option for multiple myeloma, immunotherapy comes in various forms that stimulate the immune system in different ways.

2023 Myeloma Treatment Algorithm Cco January 19, 2024 , by nadia jaber. daratumumab (pink) is an antibody drug that binds to specific proteins on multiple myeloma cells (blue). it kills cancer cells directly and also recruits immune cells that kill cancer cells. a large randomized clinical trial for people newly diagnosed with multiple myeloma has found that adding the drug. Dimopoulos ma, moreau p, terpos e, et al. multiple myeloma: eha esmo clinical practice guidelines for diagnosis, treatment and follow up. hemasphere 2021;5(2):e528 e528. pubmed. Velcade is a newer type of therapy known as proteasome inhibitor. it works by disrupting cellular processes and causing death of cancer cells. it is currently approved for the treatment of multiple myeloma in patients who have received prior therapy. the added indication for velcade includes both transplant eligible and ineligible patients, and. Bortezomib, lenalidomide, and dexamethasone (vrd) is a preferred first line treatment option for patients with newly diagnosed multiple myeloma. whether the addition of the anti cd38 monoclonal ant.
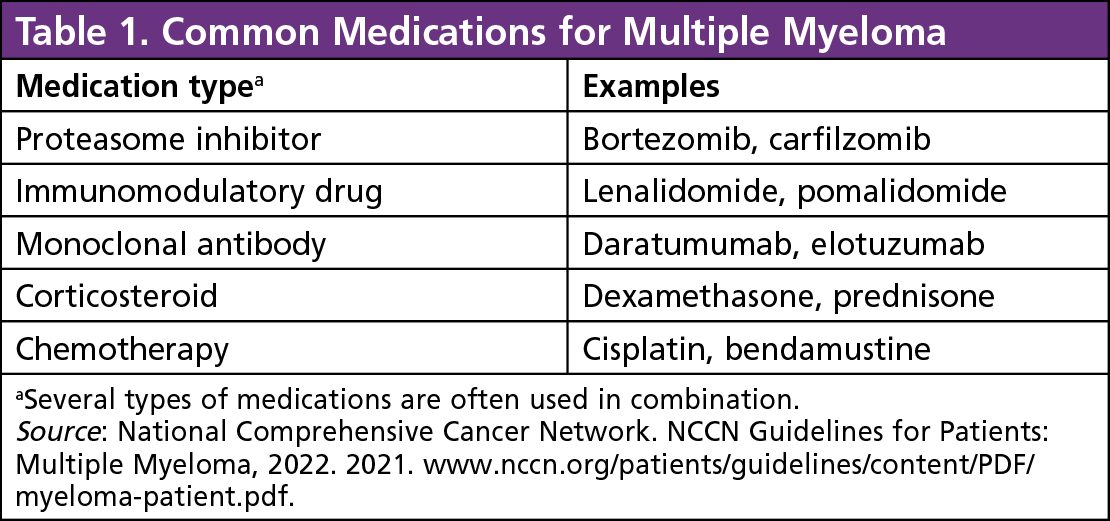
Lambda Light Chain Multiple Myeloma Icd 10 Americanwarmoms Org Velcade is a newer type of therapy known as proteasome inhibitor. it works by disrupting cellular processes and causing death of cancer cells. it is currently approved for the treatment of multiple myeloma in patients who have received prior therapy. the added indication for velcade includes both transplant eligible and ineligible patients, and. Bortezomib, lenalidomide, and dexamethasone (vrd) is a preferred first line treatment option for patients with newly diagnosed multiple myeloma. whether the addition of the anti cd38 monoclonal ant. Credit: penn medicine. a type of immunotherapy called car t cell therapy is now an option for some people with multiple myeloma. on march 26, the food and drug administration (fda) approved idecabtagene vicleucel (abecma) for people with multiple myeloma that has not responded to or has returned after at least four different prior cancer. The treatment of relapsed refractory multiple myeloma (mm) has evolved to include several new options. these include new combinations with second generation proteasome inhibitors (pi); second generation immunomodulators, monoclonal antibodies, car t cells, bispecific antibodies, selinexor, venetoclax, and many others. most patients with mm undergo several cycles of remissions and relapse, and.

Comments are closed.