Orbital Diagrams And Electron Configuration Basic Introduction Chemistry Practice Problems

Orbital Diagrams And Electron Configuration Basic Introduction An electron configuration is a method of indicating the arrangement of electrons about a nucleus. a typical electron configuration consists of numbers, letters, and superscripts with the following format: a number indicates the energy level (the number is called the principal quantum number.). a letter indicates the type of orbital; s, p, d, f. This online quiz is intended to give you extra practice in writing electron configurations for each of the first 102 chemical elements. this quiz aligns with the following ngss standard (s): hs ps1 1, hs ps1 2. select your preferences below and click 'start' to give it a try! number of problems: 1. 5.

Electron Configuration Practice Worksheet This chemistry video tutorial provides a basic introduction into orbital diagrams and electron configuration. it explains how to write the orbital diagram n. Problem 3.1.12 3.1. 12. in one area of australia, the cattle did not thrive despite the presence of suitable forage. an investigation showed the cause to be the absence of sufficient cobalt in the soil. cobalt forms cations in two oxidation states, co 2 and co 3 . write the electron structure of the two cations. Which element is depicted from this orbital diagram. electrons occupy orbitals of lowest energy first is part of what electron configuration rule? an atomic orbital can only hold a maximum of 2 electrons, each with opposite spins. an atomic orbital can hold a minimum of 6 electrons, each with opposite spins. an atomic orbital can hold a maximum. This chemistry video tutorial provides a basic introduction into electron configuration. it contains plenty of practice problems including the electron conf.

Orbital Diagrams And Electron Configuration Worksheet Answer Which element is depicted from this orbital diagram. electrons occupy orbitals of lowest energy first is part of what electron configuration rule? an atomic orbital can only hold a maximum of 2 electrons, each with opposite spins. an atomic orbital can hold a minimum of 6 electrons, each with opposite spins. an atomic orbital can hold a maximum. This chemistry video tutorial provides a basic introduction into electron configuration. it contains plenty of practice problems including the electron conf. This video is a great way to practice finding the complete electron configuration, the condensed electron configuration, the orbital diagrams, the valence el. In this case, 2 2 6 2 6 2 10 6 2 1= 39 and z=39, so the answer is correct. a slightly more complicated example is the electron configuration of bismuth (symbolized bi, with z = 83). the periodic table gives the following electron configuration: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p65s2 4d10 5p6 6s2 4f14 5d10 6p3.
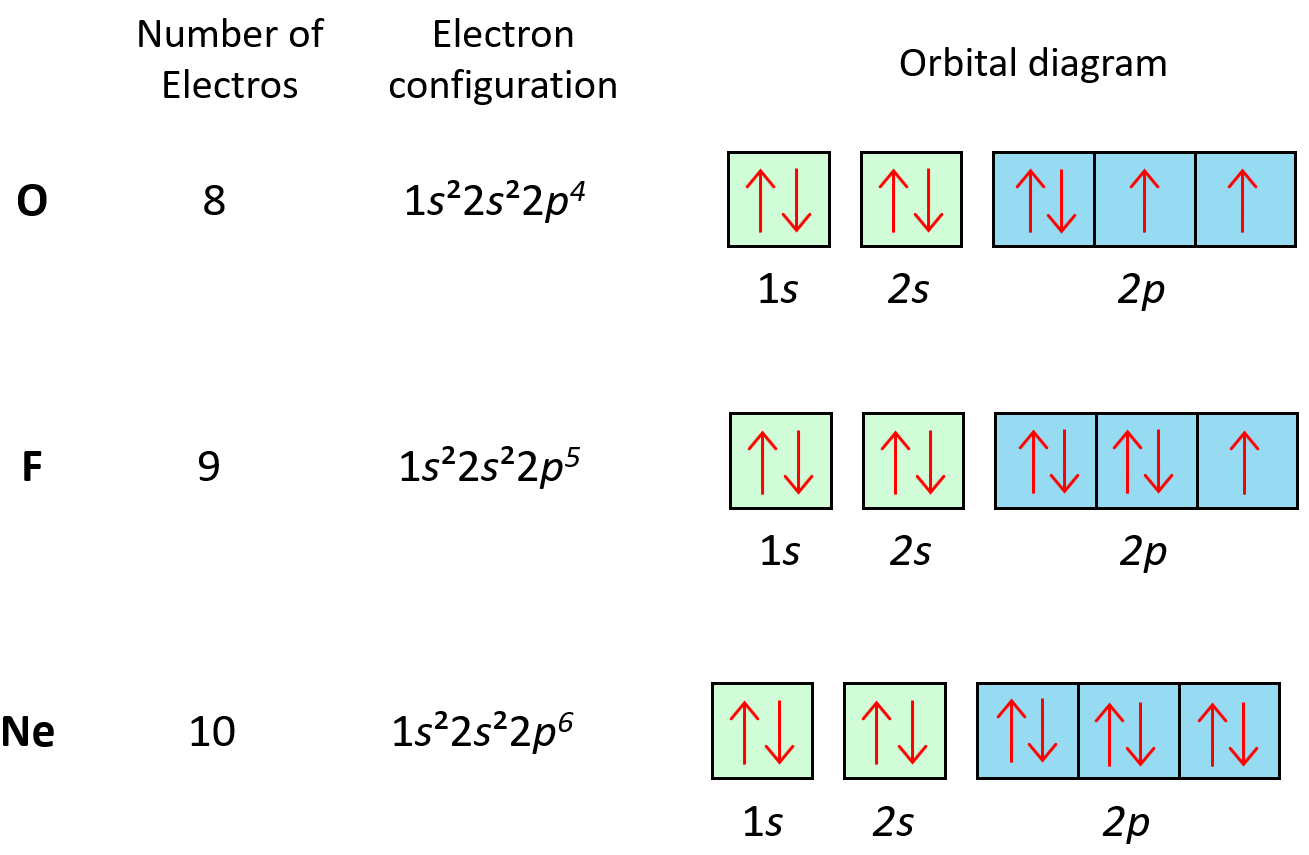
Electron Configuration Diagram Orbitals This video is a great way to practice finding the complete electron configuration, the condensed electron configuration, the orbital diagrams, the valence el. In this case, 2 2 6 2 6 2 10 6 2 1= 39 and z=39, so the answer is correct. a slightly more complicated example is the electron configuration of bismuth (symbolized bi, with z = 83). the periodic table gives the following electron configuration: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p65s2 4d10 5p6 6s2 4f14 5d10 6p3.

Comments are closed.