Orbital Diagrams Chemistry Steps
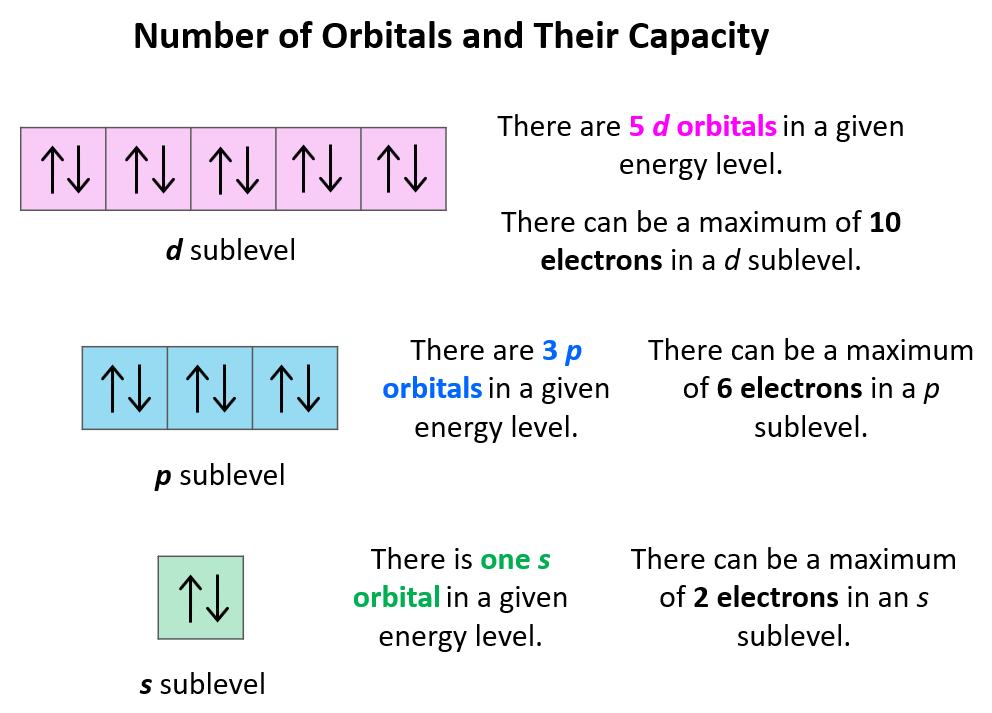
Orbital Diagrams Chemistry Steps Electronic structure of atoms. orbital diagrams. in orbitals diagrams, the orbitals are shown as boxes, and the electrons in them as arrows pointing up or down. each box represents one orbital, and each arrow indicates one electron. this is a way of showing the electron configuration of the atom. for example, the orbital diagram of li can be. To allow space for the next step, draw the orbitals further apart than you would for an atomic orbital diagram. in the second (center) column, draw lines above and below each orbital. the bottom line(s) represent the bonding orbital(s), and the top line(s) represent the antibonding orbital(s).
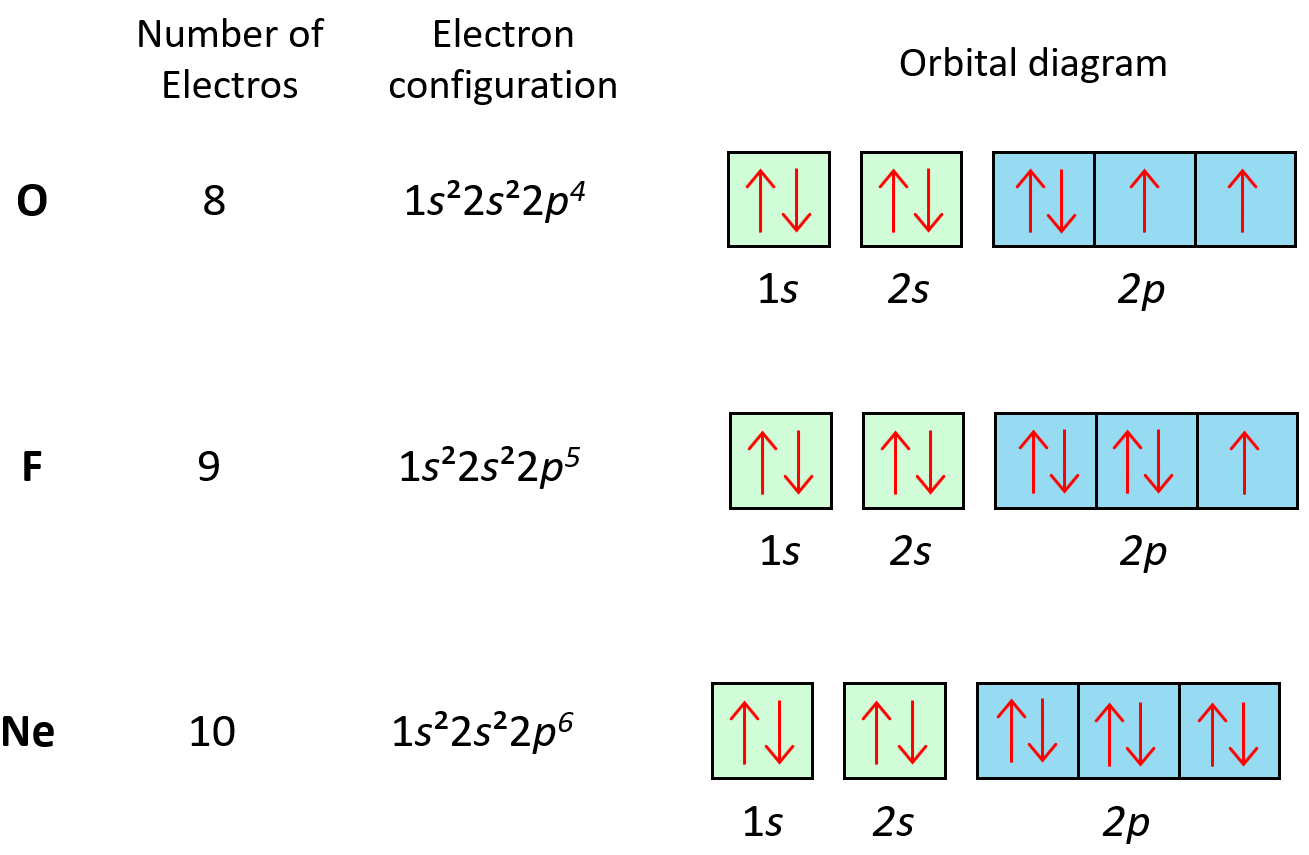
Orbital Diagrams Chemistry Steps In this case, 2 2 6 2 6 2 10 6 2 1= 39 and z=39, so the answer is correct. a slightly more complicated example is the electron configuration of bismuth (symbolized bi, with z = 83). the periodic table gives the following electron configuration: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p65s2 4d10 5p6 6s2 4f14 5d10 6p3. Molecular orbital diagrams. this scheme of bonding and antibonding orbitals is usually depicted by a molecular orbital diagram such as the one shown here for the dihydrogen ion h2 . atomic valence electrons (shown in boxes on the left and right) fill the lower energy molecular orbitals before the higher ones, just as is the case for atomic. The 2s orbital is shown below, once again represented by a dot density diagram and a boundary surface diagram. notice how the dot density diagram reveals a feature about the 2 s orbital that boundary surface does not: a node divides the 2 s orbital in two, a portion of the electron cloud is near the center, while another portion lies beyond the node (the circular region with no dots). An orbital box diagram can be written as well. boxes, or horizontal lines represent the orbitals, arrows represent the electrons, and if an orbital is full, the electrons must be of opposite spin–one arrow pointing up and the other one pointing down. the orbital box diagrams are listed for the first 20 elements in the figure below.
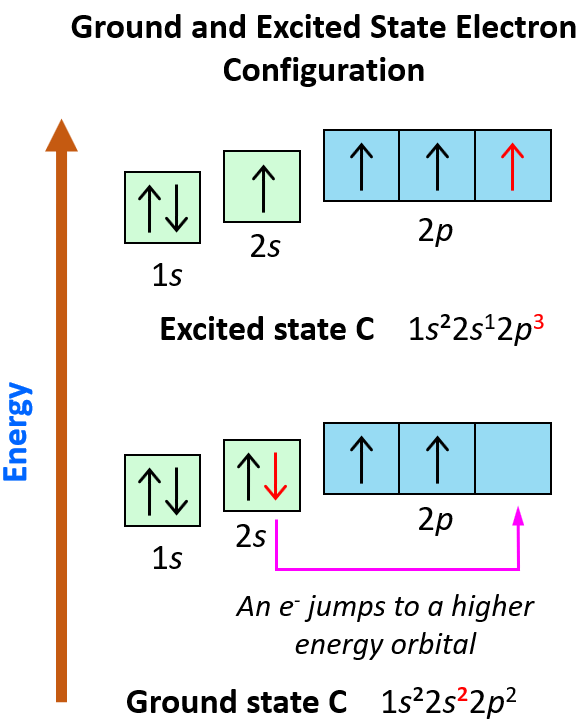
Orbital Diagrams Chemistry Steps The 2s orbital is shown below, once again represented by a dot density diagram and a boundary surface diagram. notice how the dot density diagram reveals a feature about the 2 s orbital that boundary surface does not: a node divides the 2 s orbital in two, a portion of the electron cloud is near the center, while another portion lies beyond the node (the circular region with no dots). An orbital box diagram can be written as well. boxes, or horizontal lines represent the orbitals, arrows represent the electrons, and if an orbital is full, the electrons must be of opposite spin–one arrow pointing up and the other one pointing down. the orbital box diagrams are listed for the first 20 elements in the figure below. The first two electrons in lithium fill the 1s orbital and have the same sets of four quantum numbers as the two electrons in helium. the remaining electron must occupy the orbital of next lowest energy, the 2s orbital (figure 6.26 or figure 6.27). thus, the electron configuration and orbital diagram of lithium are:. Steps for drawing an orbital diagram. draw a long vertical arrow that points upward. label the arrow energy. the arrow shows a qualitative representation of increasing orbital energy. write out the electron configuration to determine which orbitals are filled. remember, we can use the periodic table to help us.
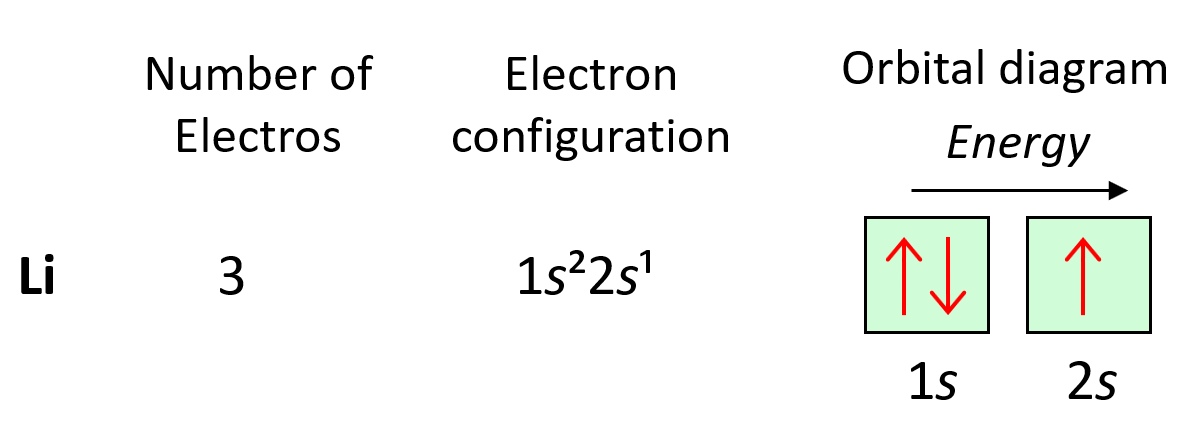
Orbital Diagrams Chemistry Steps The first two electrons in lithium fill the 1s orbital and have the same sets of four quantum numbers as the two electrons in helium. the remaining electron must occupy the orbital of next lowest energy, the 2s orbital (figure 6.26 or figure 6.27). thus, the electron configuration and orbital diagram of lithium are:. Steps for drawing an orbital diagram. draw a long vertical arrow that points upward. label the arrow energy. the arrow shows a qualitative representation of increasing orbital energy. write out the electron configuration to determine which orbitals are filled. remember, we can use the periodic table to help us.

Comments are closed.