Outcomes And Endpoints In Cancer Trials Bridging The Divide The

Pdf Outcomes And Endpoints In Cancer Trials Bridging The Divide Cancer is not one disease. outcomes and endpoints in trials should incorporate the therapeutic modality and cancer type because these factors affect clinician and patient expectations. in this review, we discuss how to: define the importance of endpoints; make endpoints understandable to patients; improve the use of patient reported outcomes. Introduction. the key priorities in the treatment of cancer are to enable affected individuals to live longer or better, and ideally both, than they would without therapy. 1 the discussion of outcomes and endpoints in oncology trials needs to take into account cancer type and therapeutic modality as these parameters affect the expectations of both clinicians and patients.

Figure 1 From Outcomes And Endpoints In Trials Of Cancer Treatment The Outcomes and endpoints in cancer trials: bridging the divide. cancer is not one disease. outcomes and endpoints in trials should incorporate the therapeutic modality and cancer type because these factors aff ect clinician and patient expectations. in this review, we discuss how to: defi ne the importance of endpoints; make endpoints. In this review, we discuss how to: define the importance of endpoints; make endpoints understandable to patients; improve the use of patient reported outcomes; advance endpoints to parallel changes in trial design and therapeutic interventions; and integrate these improvements into trials and practice. endpoints need to reflect benefit to. The primary goal of cancer drug therapy is to prolong life or to improve quality of life. 1, 2 overall survival (os) is the most reliable clinical trial end point to inform regulatory approvals of. Doi: 10.1016 s1470 2045(14)70380 8 corpus id: 29540270; outcomes and endpoints in cancer trials: bridging the divide. @article{wilson2015outcomesae, title={outcomes and endpoints in cancer trials: bridging the divide.}, author={michelle k wilson and deborah e. collyar and diana t. chingos and michael l. friedlander and tony w. ho and katherine karakasis and stanley b. kaye and mahesh k. b.
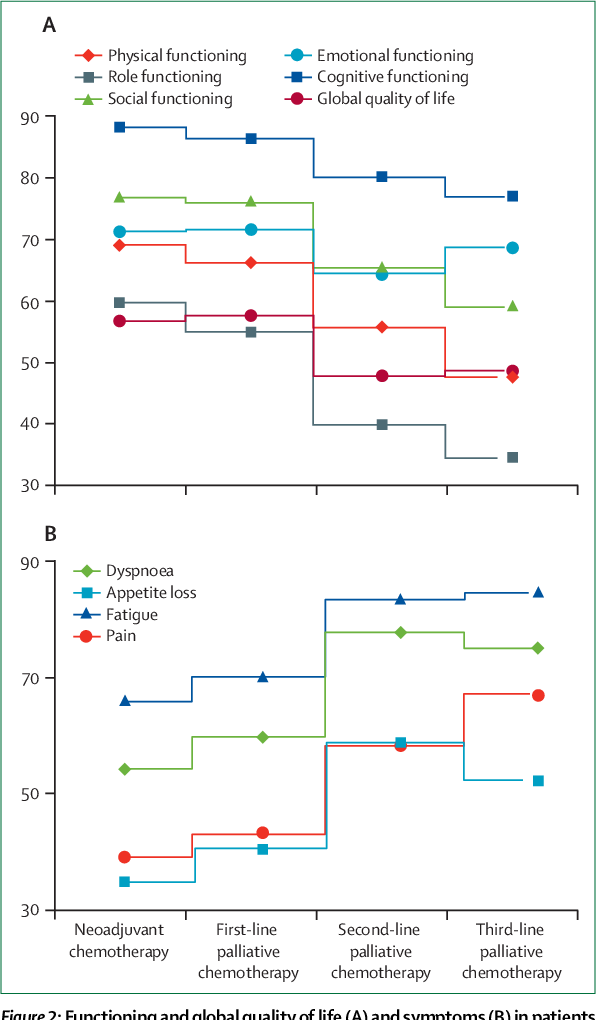
Figure 2 From Outcomes And Endpoints In Trials Of Cancer Treatment The The primary goal of cancer drug therapy is to prolong life or to improve quality of life. 1, 2 overall survival (os) is the most reliable clinical trial end point to inform regulatory approvals of. Doi: 10.1016 s1470 2045(14)70380 8 corpus id: 29540270; outcomes and endpoints in cancer trials: bridging the divide. @article{wilson2015outcomesae, title={outcomes and endpoints in cancer trials: bridging the divide.}, author={michelle k wilson and deborah e. collyar and diana t. chingos and michael l. friedlander and tony w. ho and katherine karakasis and stanley b. kaye and mahesh k. b. Alternative endpoints such as progression free survival, disease free survival, and objective response rate have been used to identify benefit earlier, but their true validity as surrogate endpoints is controversial. in this review we discuss the measurement, assessment, and benefits and limitations of trial endpoints in use for cancer treatment. Academia.edu is a platform for academics to share research papers. outcomes and endpoints in cancer trials: bridging the divide (pdf) outcomes and endpoints in cancer trials: bridging the divide | tony w ho academia.edu.

Pdf Intermediate Endpoints As Surrogates For Outcomes In Cancer Alternative endpoints such as progression free survival, disease free survival, and objective response rate have been used to identify benefit earlier, but their true validity as surrogate endpoints is controversial. in this review we discuss the measurement, assessment, and benefits and limitations of trial endpoints in use for cancer treatment. Academia.edu is a platform for academics to share research papers. outcomes and endpoints in cancer trials: bridging the divide (pdf) outcomes and endpoints in cancer trials: bridging the divide | tony w ho academia.edu.

Outcomes And Endpoints In Cancer Trials Bridging The Divide The

Comments are closed.