Parp Inhibitors And The Embraca Study Of Talazoparib In Brca Related Metastatic Breast Cancer

Study Shows How Parp Inhibitors Can Be Empowered In Breast Cancer In the phase 2 abrazo study (clinicaltrials.gov number, nct02034916), talazoparib also had single agent activity in two cohorts of patients with metastatic breast cancer and a germline brca1 2. In breast cancer, two parp inhibitors, olaparib and talazoparib, have been approved for treatment of gbrcam carriers with metastatic her2 negative breast cancer based on the olympiad and embraca.
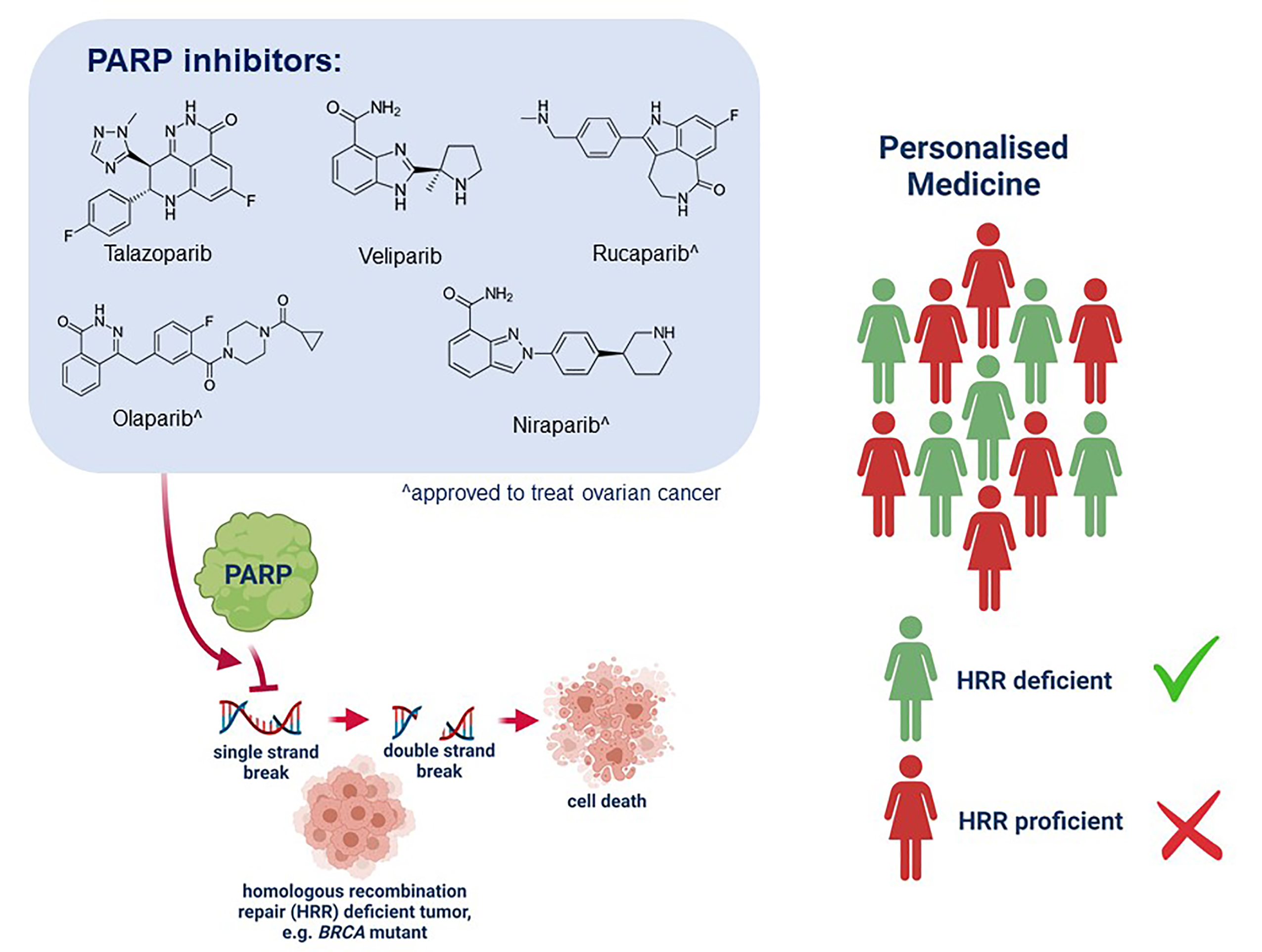
Ijms Free Full Text Parp Inhibitors Display Differential Efficacy The introduction of parp inhibitors has revolutionized the management and treatment of patients with pathogenic germline variants of brca1 2 who have developed breast cancer. the implementation of parp inhibitors in clinical settings can be challenging due to their overlapping indications with other drugs, including both recently approved. Talazoparib, a parp inhibitor, is active in germline brca1 and brca2 (gbrca1 2) mutant advanced breast cancer, but its activity beyond gbrca1 2 is poorly understood. we conducted talazoparib. Breast cancer is one of the tumors with the highest prevalence rate among women in the world, and its brca1 2 gene is a common mutation site. talazoparib, as a targeted parp inhibitor, can. Less than 5% of patients with metastatic breast cancer harbor gbrca mutations. 3 in october 2018, the fda approved the parp inhibitor talazoparib for the treatment of adult patients with.
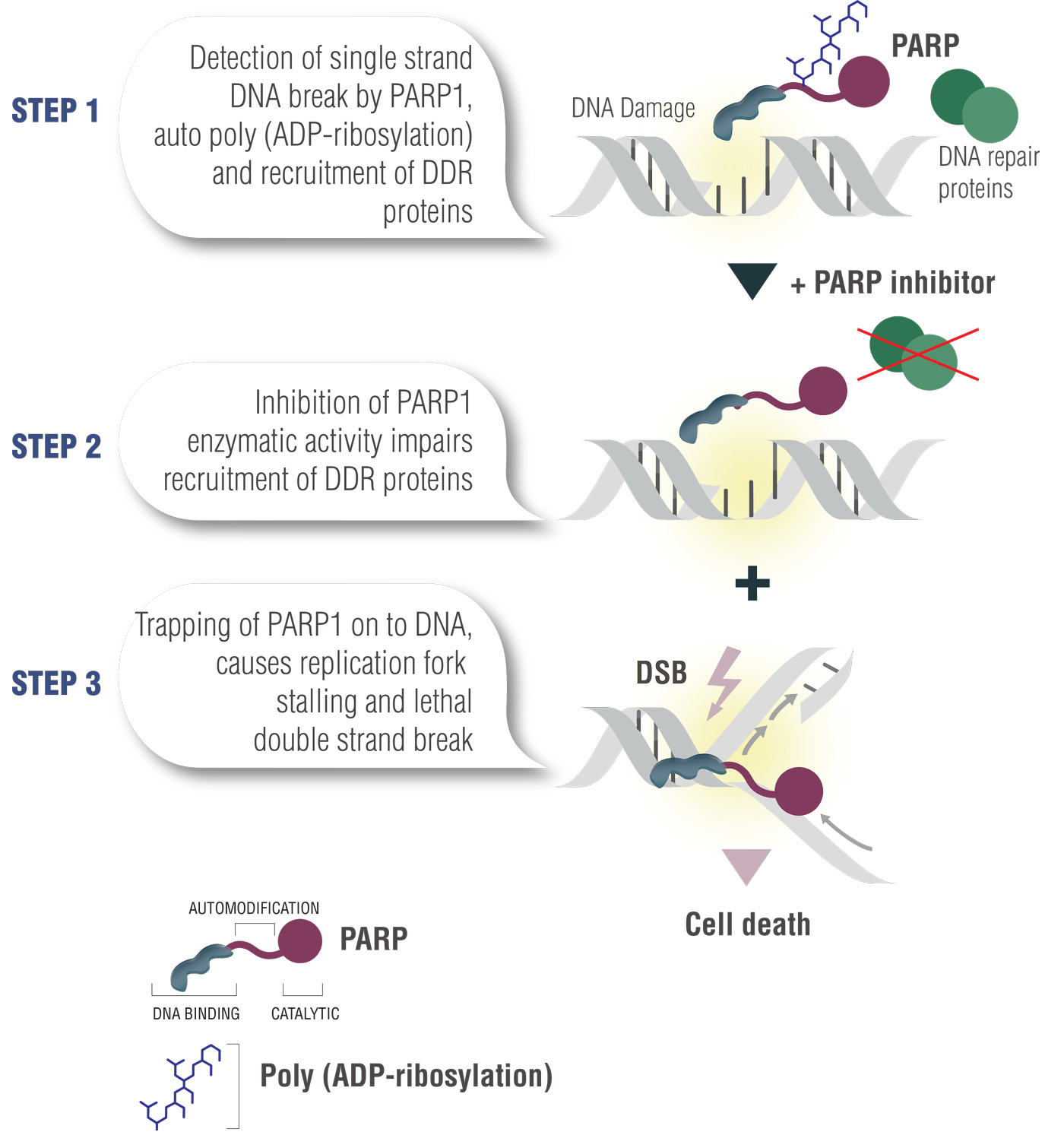
Mode Of Action Oncologypro Breast cancer is one of the tumors with the highest prevalence rate among women in the world, and its brca1 2 gene is a common mutation site. talazoparib, as a targeted parp inhibitor, can. Less than 5% of patients with metastatic breast cancer harbor gbrca mutations. 3 in october 2018, the fda approved the parp inhibitor talazoparib for the treatment of adult patients with. Methods: we conducted a randomized, open label, phase 3 trial in which patients with advanced breast cancer and a germline brca1 2 mutation were assigned, in a 2:1 ratio, to receive talazoparib (1 mg once daily) or standard single agent therapy of the physician's choice (capecitabine, eribulin, gemcitabine, or vinorelbine in continuous 21 day. Abstract. loss of function mutations in brca1 and brca2 are detected in at least 5% of unselected patients with breast cancer (bc). these bc susceptibility genes encode proteins critical for dna homologous recombination repair (hrr). this review provides an update on oral poly (adp ribose) polymerase (parp) inhibitors for the treatment of bc.

Comments are closed.