Particulate Nature Of Matter And Changes Of State
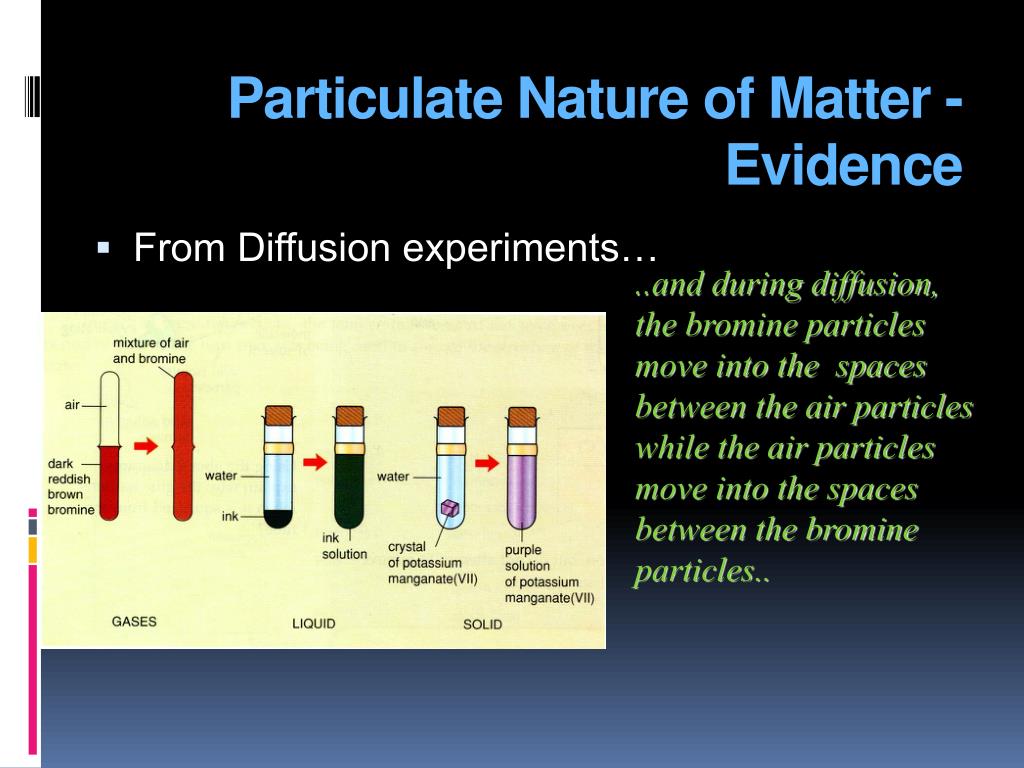
Ppt The Particulate Nature Of Matter Powerpoint Presentation Free About press copyright contact us creators advertise developers terms privacy policy & safety how works test new features nfl sunday ticket press copyright. As temperature increases, particles of matter move faster. when the temperature rises, the kinetic energy of the particles rises, and they begin to vibrate. as a result, they move quickly, weakening the forces of attraction between the particles. this can eventually result in a change in the state of matter. read more: change in the states of.

Particulate Nature Of Matter Classnotes Ng The structure of matter at the sub microscopic level, and the forces between particles, underlies the explanation of macroscopic properties. during 11–14, students gain some appreciation of the nature of the particles (atoms, ions, molecules). at 14–16, you will expand on how these different particles are formed and how this affects the. There is also a quick experiment you can do at home to prove that air is matter. gcse physics particle theory & states of matter #25 (2020) this video (4:33 min.) from cognito covers particle theory, how substances change from one state to another, and the idea that density doesn't change when substances change state. Structure 1.1—introduction to the particulate nature of matter (sl hl) structure 1.1.1 elements are the primary constituents of matter, which cannot be chemically broken down into simpler substances. structure 1.1.2 —the kinetic molecular theory is a model to explain physical properties of matter (solids, liquids and gases) and changes of. 2. develop and use models to describe the nature of matter; demonstrate how they provide a simple way to to account for the conservation of mass, changes of state, physical change, chemical change, mixtures, and their separation. 4. classify substances as elements, compounds, mixtures, metals, non metals, solids, liquids, gases and solutions.

Particulate Nature Of Matter And Changes Of State Animated Gif Structure 1.1—introduction to the particulate nature of matter (sl hl) structure 1.1.1 elements are the primary constituents of matter, which cannot be chemically broken down into simpler substances. structure 1.1.2 —the kinetic molecular theory is a model to explain physical properties of matter (solids, liquids and gases) and changes of. 2. develop and use models to describe the nature of matter; demonstrate how they provide a simple way to to account for the conservation of mass, changes of state, physical change, chemical change, mixtures, and their separation. 4. classify substances as elements, compounds, mixtures, metals, non metals, solids, liquids, gases and solutions. states of matter phet interactive simulations. Structure 1.1.1 elements are the primary constituents of matter, which cannot be chemically broken down into simpler substances. structure 1.1.2—the kinetic molecular theory is a model to explain physical properties of matter (solids, liquids and gases) and changes of state. structure 1.1.3—the temperature, t , in kelvin (k) is a measure of.
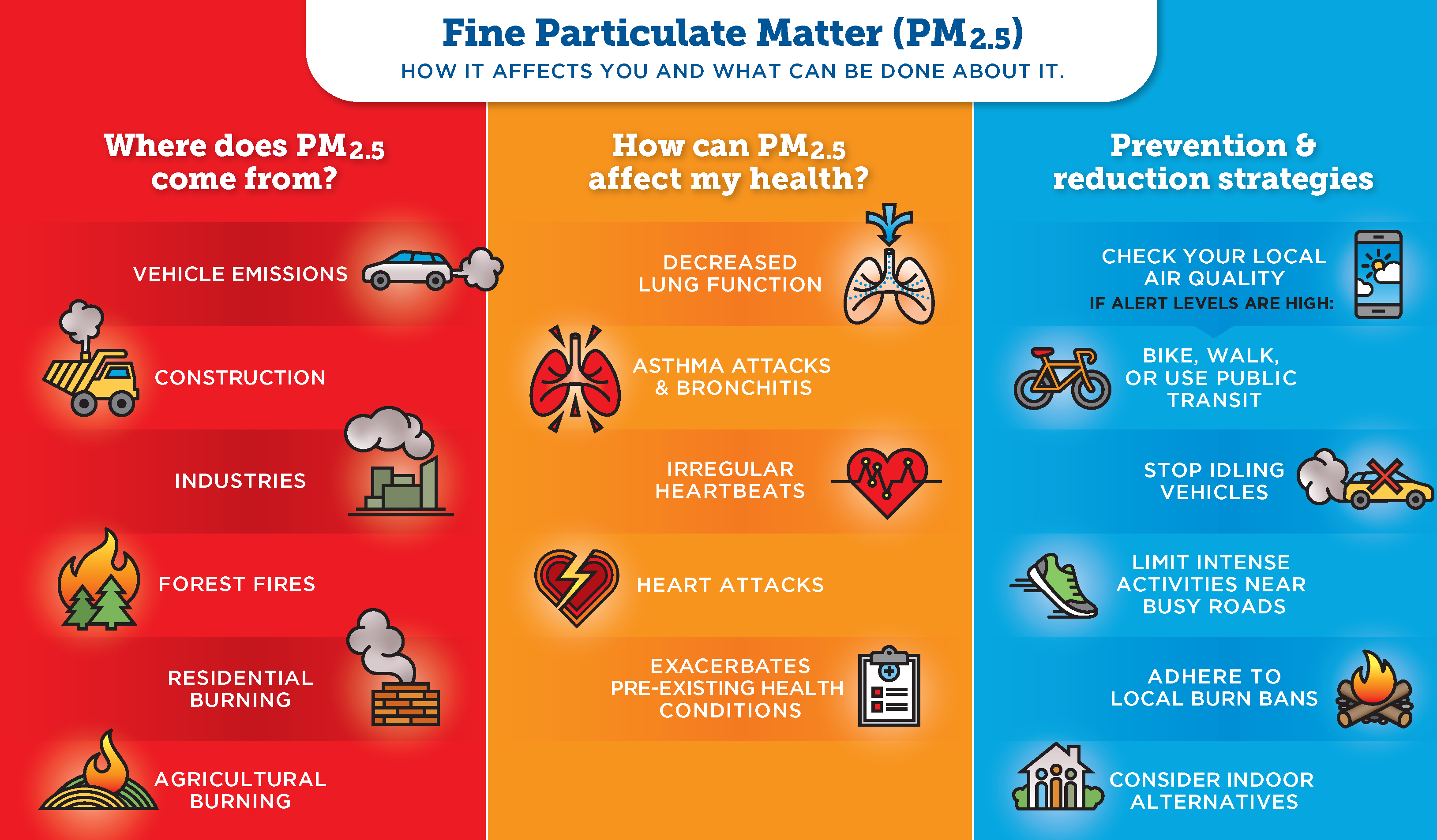
What Is Particulate Matter Air Central Texas English states of matter phet interactive simulations. Structure 1.1.1 elements are the primary constituents of matter, which cannot be chemically broken down into simpler substances. structure 1.1.2—the kinetic molecular theory is a model to explain physical properties of matter (solids, liquids and gases) and changes of state. structure 1.1.3—the temperature, t , in kelvin (k) is a measure of.

Comments are closed.