Pdf Exposureвђ Response Modeling Of Clinical End Points Using Latent

Pdf A Flexible Dose Response Modeling Framework Based On Continuous Exposure–response modeling of clinical end points using latent variable indirect response models.pdf available via license: cc by nc nd 3.0 content may be subject to copyright. Existing applications typically apply idr models directly to the end point, often using the 1 pathway approach to model the placebo effect. 18 the difference between 1 and 2 pathway models have been discussed. 3 the latent variable model derivation may be extended to this case by treating the latent variable l(t) as the observed response z(t.

Exposureвђ Response Modeling Of Clinical End Points Using Lat A general framework of applying mechanism‐based models to various types of clinical end points is described, with a focus on the indirect response models. exposure–response modeling facilitates effective dosing regimen selection in clinical drug development, where the end points are often disease scores and not physiological variables. appropriate models need to be consistent with. Accurate characterization of longitudinal exposure response of clinical trial endpoints is important in optimizing dose and dosing regimens in drug development. clinical endpoints are often categorical, for which much progress has been made recently in latent variable indirect response (idr) modeling with single drugs. however, such applications have not yet been used for trials employing. Improving the quality of exposure–response modeling is important in clinical drug development. the general joint modeling of multiple endpoints is made possible in part by recent progress on the latent variable indirect response (idr) modeling for ordered categorical endpoints. this manuscript aims to investigate, when modeling a continuous and a categorical clinical endpoint, the level of. Informative exposure–response modeling of clinical endpoints is important in drug development. there has been much recent progress in latent variable modeling of ordered categorical endpoints, including the application of indirect response (idr) models and accounting for residual correlations between multiple categorical endpoints. this manuscript describes a framework of latent variable.

Pdf Population Pharmacokinetics And Exposureвђ Response Analyses Of Improving the quality of exposure–response modeling is important in clinical drug development. the general joint modeling of multiple endpoints is made possible in part by recent progress on the latent variable indirect response (idr) modeling for ordered categorical endpoints. this manuscript aims to investigate, when modeling a continuous and a categorical clinical endpoint, the level of. Informative exposure–response modeling of clinical endpoints is important in drug development. there has been much recent progress in latent variable modeling of ordered categorical endpoints, including the application of indirect response (idr) models and accounting for residual correlations between multiple categorical endpoints. this manuscript describes a framework of latent variable. Exposure response modeling plays an important role in optimizing dose and dosing regimens during clinical drug development. the modeling of multiple endpoints is made possible in part by recent progress in latent variable indirect response (idr) modeling for ordered categorical endpoints. this manus …. Exposure–response modeling facilitates effective dosing regimen selection in clinical drug development, where the end points are often disease scores and not physiological variables. appropriate models need to be consistent with pharmacology and identifiable from the time courses of available data.
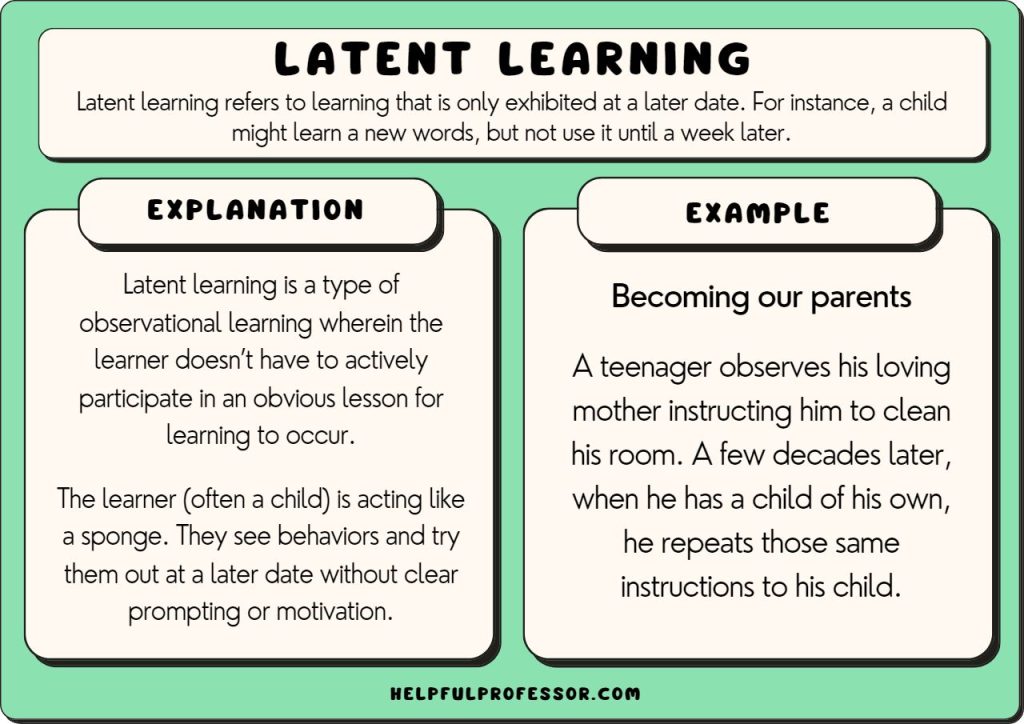
10 Latent Learning Examples 2024 Exposure response modeling plays an important role in optimizing dose and dosing regimens during clinical drug development. the modeling of multiple endpoints is made possible in part by recent progress in latent variable indirect response (idr) modeling for ordered categorical endpoints. this manus …. Exposure–response modeling facilitates effective dosing regimen selection in clinical drug development, where the end points are often disease scores and not physiological variables. appropriate models need to be consistent with pharmacology and identifiable from the time courses of available data.

Comments are closed.