Pharma 4 0 News Ispe International Society For Pharmaceutical
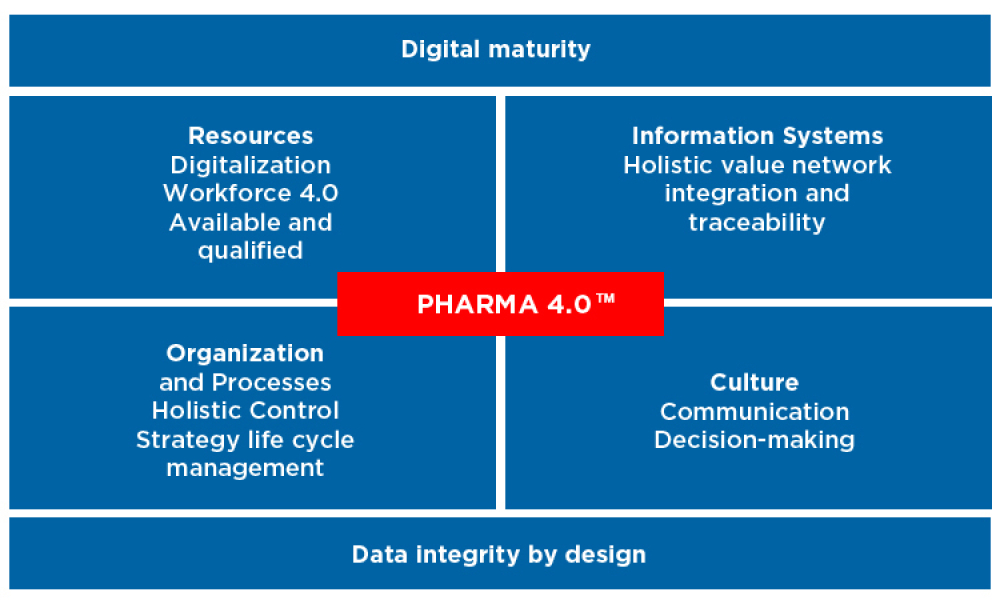
Pharma 4 0 Ispe International Society For Pharmaceutical E The ispe pharma 4.0™ special interest group (sig) launched in 2015 to provide a road map for new challenges of digitalization, industry 4.0, and the smart factory. the sig addresses how pharmaceutical industry stakeholders, including regulatory authorities, can achieve benefits from pharma 4.0™ initiatives. features. The ispe pharma 4.0™ community of practice (cop) is comprised of ispe members engaged in implementing pharma 4.0™ principles in their organizations or simply wanting to learn more about the concepts and network with other members interested in the topic. membership in the pharma 4.0™ cop is open to all ispe members.

Pharma 4 0 The Pharmaceutical Industry Looks To The Future Tecnomaco Digitalization, an important component of pharma 4.0™, will connect everything, creating new levels of transparency and adaptivity for a “smart” plant floor. this will enable faster decision making, and provide in line and on time control over business, operations, quality, and regulatory compliance. notably, this new connectedness will. The international society for pharmaceutical engineering (ispe) and its members are developing the roadmap to introduce industry 4.0 at the pharmaceutical industry. pharma 4.0 is defined as an operating model based on the industry 4.0 elements, resources, information systems, organization processes and culture. The ispe sig pharma 4.0 worked since 2017 on concepts around the digital transformation process, the implementation methodologies & roadmap, and a pharma 4.0 operating model for pharma organizations. the pharma 4.0 baseline guide will summarize and describe the structured approach for a digital transformational process, including practical use. The pharma 4.0™ survey is at its 6th edition and the aim is to get the ispe pharma 4.0 sig yearly update on the state of the art, the goals, the mindset and also the sentiment about 4.0 from the life science protagonists.previous editions have been carried out since 2017, having always around 300 to 400 respondents.
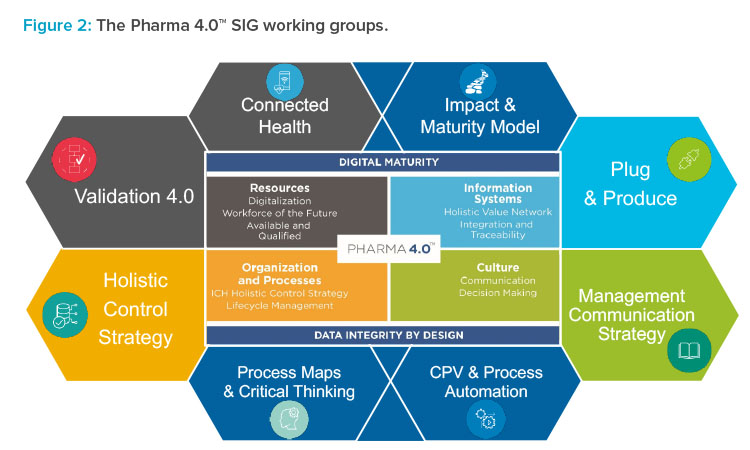
Ispe Pharma 4 0в ў Sig Its Working Groups Pharmaceutical Engineering The ispe sig pharma 4.0 worked since 2017 on concepts around the digital transformation process, the implementation methodologies & roadmap, and a pharma 4.0 operating model for pharma organizations. the pharma 4.0 baseline guide will summarize and describe the structured approach for a digital transformational process, including practical use. The pharma 4.0™ survey is at its 6th edition and the aim is to get the ispe pharma 4.0 sig yearly update on the state of the art, the goals, the mindset and also the sentiment about 4.0 from the life science protagonists.previous editions have been carried out since 2017, having always around 300 to 400 respondents. However, moving to pharma 4.0™, a different approach in which the data life cycle is centralized, will lead to a data centric approach. each data life cycle needs to be completed and aligned to be considered a data set. in current production processes different data sets are created for each process. visualizing the data sets amongst. Ispe baseline® guide: volume 8 – pharma 4.0™. applying emerging and digital technologies can lead to more robust and flexible manufacturing processes. this can help the pharmaceutical industry respond to drug shortages, reduce interruptions in the production and delivery of medicines, and ensure consistent clinical performance of products.
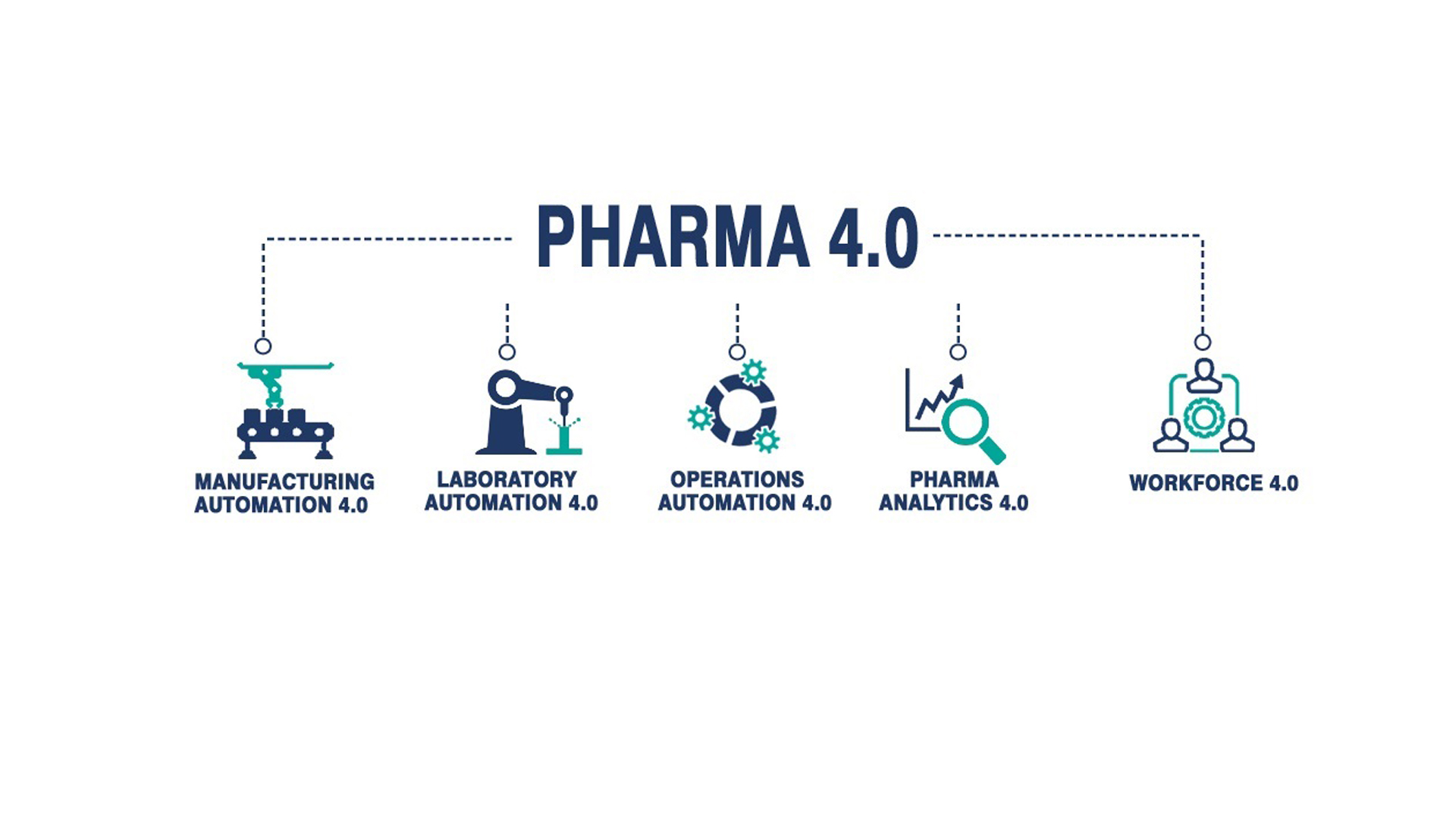
Pharma 4 0 Industry To Spend 4 5 Billion On Digital Transformation By However, moving to pharma 4.0™, a different approach in which the data life cycle is centralized, will lead to a data centric approach. each data life cycle needs to be completed and aligned to be considered a data set. in current production processes different data sets are created for each process. visualizing the data sets amongst. Ispe baseline® guide: volume 8 – pharma 4.0™. applying emerging and digital technologies can lead to more robust and flexible manufacturing processes. this can help the pharmaceutical industry respond to drug shortages, reduce interruptions in the production and delivery of medicines, and ensure consistent clinical performance of products.

Comments are closed.