Phase Change Diagram Worksheet Earthium
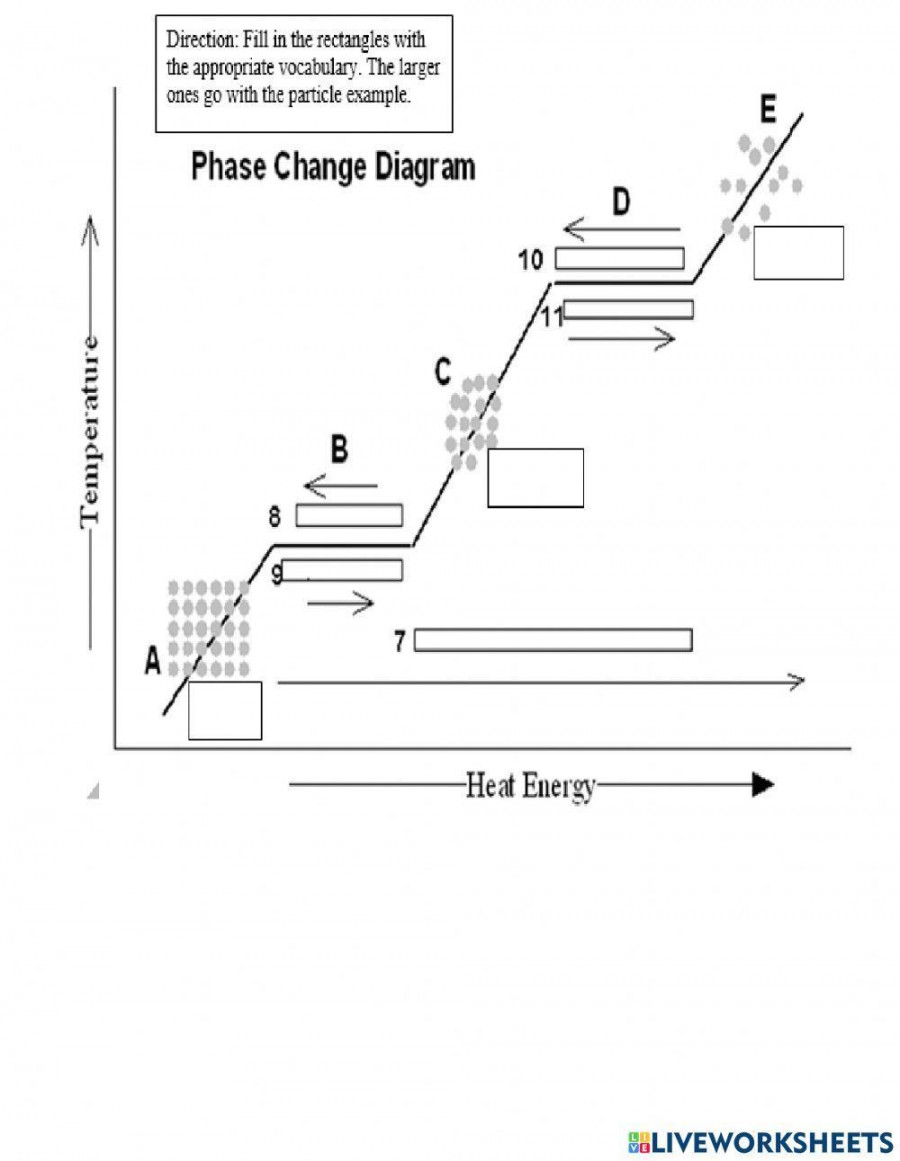
Phase Changes Diagram Worksheet вђ Martin Lindelof This heat energy is called the latent heat of fusion. between 9 and 13 minutes, the added energy increases the temperature of the substance. during the time from point d to point e, the liquid is vaporizing. by point e, the substance is completely in the gas. phase. material in this phase has infinite volume and infinite shape. Phase changes worksheet #3 l3 3 1b. since heat is still being added to the substance between points b c and d e, what is the heat doing? 2a. calculate the slope between points c d 2b. put the slope calculated in 2a, above, into words by filling in the blanks: from point c to point d on the phase change diagram, as time.

Phase Change Diagram Diagram Quizlet Unit 2: phase changes worksheets phases of matter: solid matter that has definite volume and shape. the molecules are packed together tightly and move slowly. liquid matter that has definite volume but not shape. since the molecules of a liquid are loosely packed and move with greater speed, a liquid can flow and spread out. gas. Worksheet: phase changes important: chemical chagnes are frequently accompanied by p c . for example: hydrogen gas and oxygen gas react to from liquid water. in this chemical change, something new (water) is formed, but a change of state (physical change) has also occured as a result. A phase change diagram shows the different states of matter and the temperatures and pressures that those changes occur at. triple point – the point on a phase diagram at which the three states of matter: gas, liquid, and solid coexist; critical point – the point on a phase diagram at which the substance is indistinguishable between liquid. Definitions: in a sealed container the conditions of pressure and temperature at which two phases exists in equilibrium are indicated on a phase diagram by a line. eparating the phases.triple point describes the only set of conditions at which all three phases can exist in equilibrium with one another critical point state where distinct.

Phase Change Diagram Worksheet Earthium Vrogue Co A phase change diagram shows the different states of matter and the temperatures and pressures that those changes occur at. triple point – the point on a phase diagram at which the three states of matter: gas, liquid, and solid coexist; critical point – the point on a phase diagram at which the substance is indistinguishable between liquid. Definitions: in a sealed container the conditions of pressure and temperature at which two phases exists in equilibrium are indicated on a phase diagram by a line. eparating the phases.triple point describes the only set of conditions at which all three phases can exist in equilibrium with one another critical point state where distinct. 24 06 2021. country code: ph. country: philippines. school subject: science (1061951) main content: changes in phase of matter (1415612) from worksheet author: identifying phase changes. other contents: physical change. Purpose: changes of state (phase changes) occur in the change between solid and liquid and liquid and gas. in the phase change simulation you observe a change between liquid and gas. in this activity, you will observe what happens as lauric acid (c12h24o2) melts. you will measure the temperature at timed intervals as the lauric acid is heated.
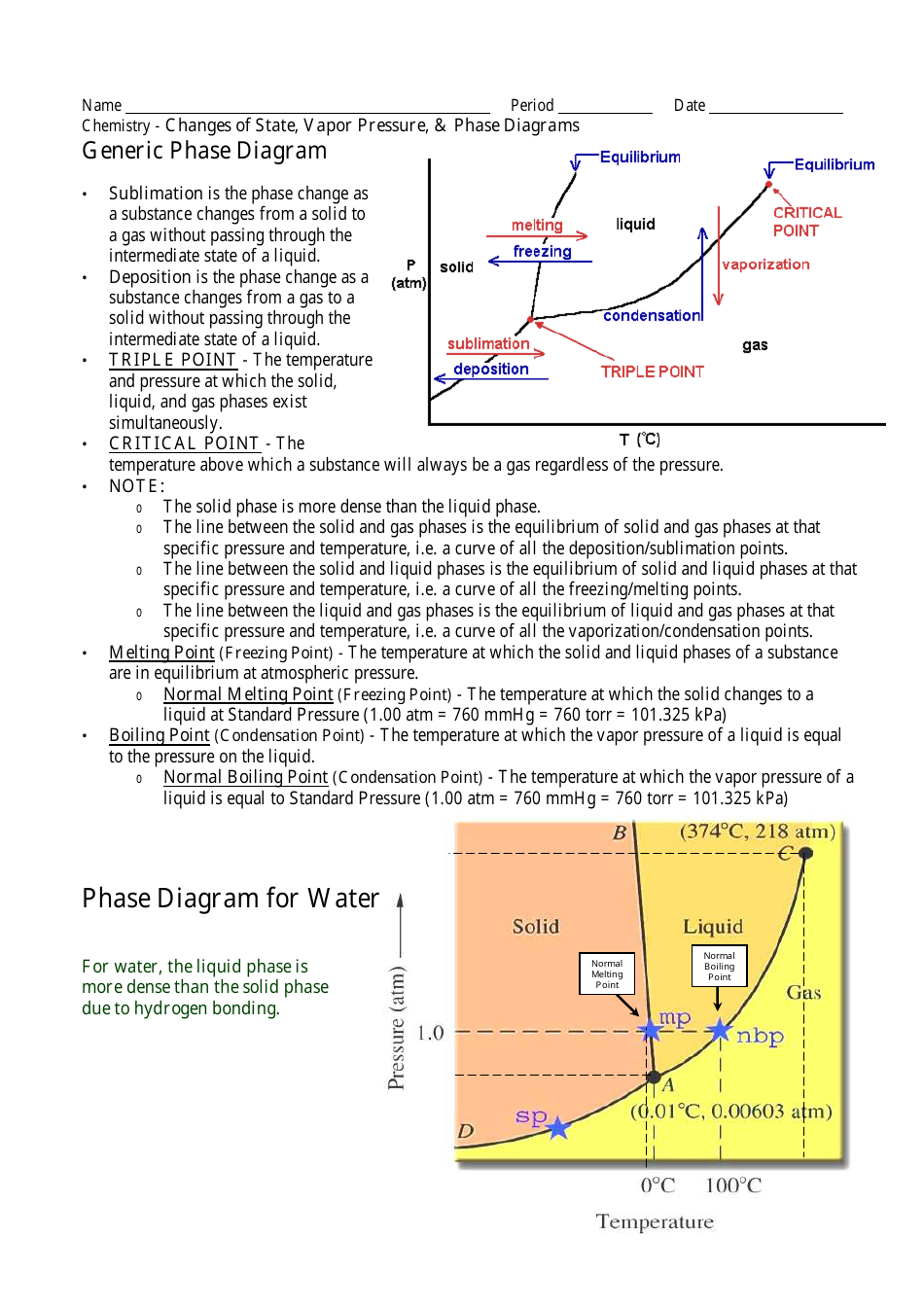
Phase Diagram Worksheet Answer Key 12 4 Phase Diagram Vrogue Co 24 06 2021. country code: ph. country: philippines. school subject: science (1061951) main content: changes in phase of matter (1415612) from worksheet author: identifying phase changes. other contents: physical change. Purpose: changes of state (phase changes) occur in the change between solid and liquid and liquid and gas. in the phase change simulation you observe a change between liquid and gas. in this activity, you will observe what happens as lauric acid (c12h24o2) melts. you will measure the temperature at timed intervals as the lauric acid is heated.

Comments are closed.