Phase Change Diagrams
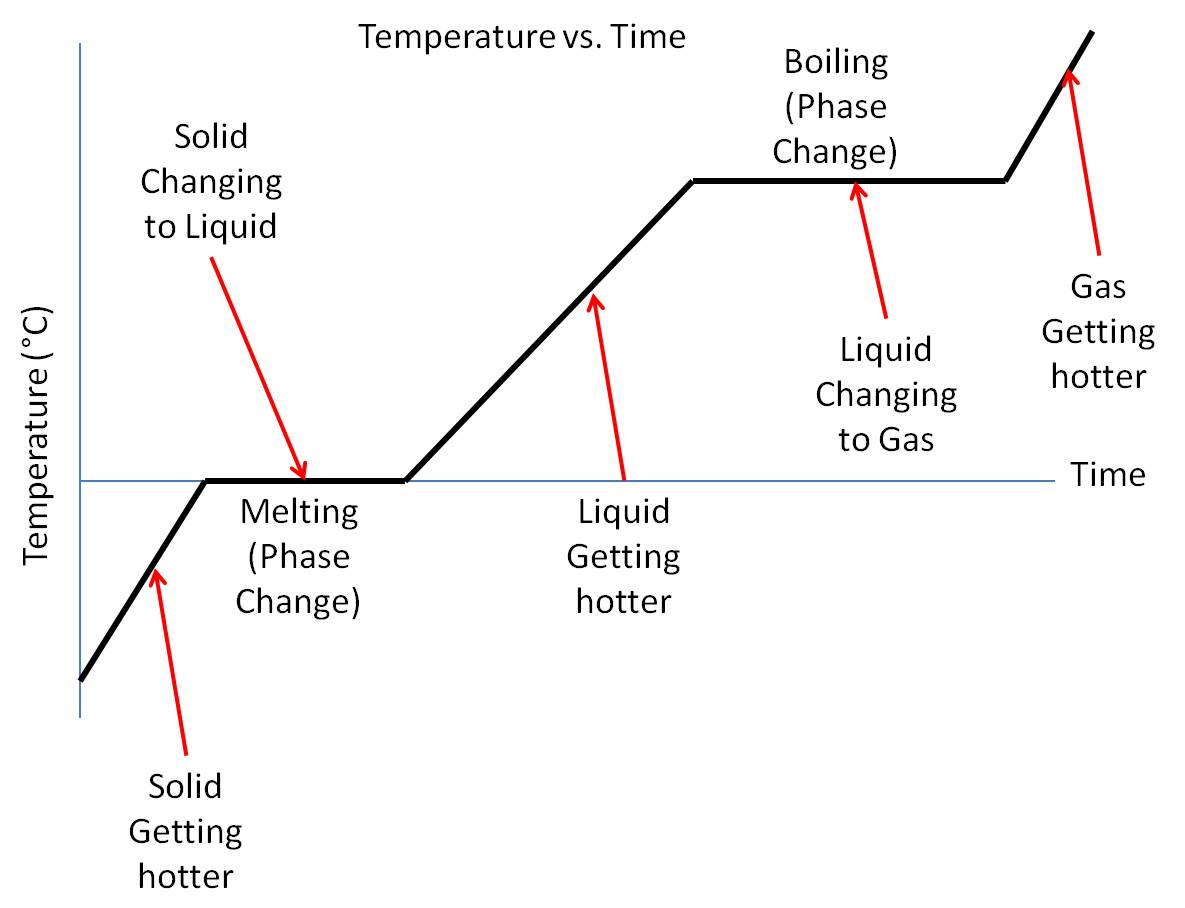
Phase Transition вђ Physics Says What Learn how to read and interpret phase diagrams, which show the physical states of a substance under different conditions of temperature and pressure. find out the definitions and examples of phase transitions, triple point, critical point, and sublimation. Learn about the six or eight phase changes of matter between solids, liquids, gases, and plasma. find out how temperature and pressure affect phase transitions and see a phase diagram.
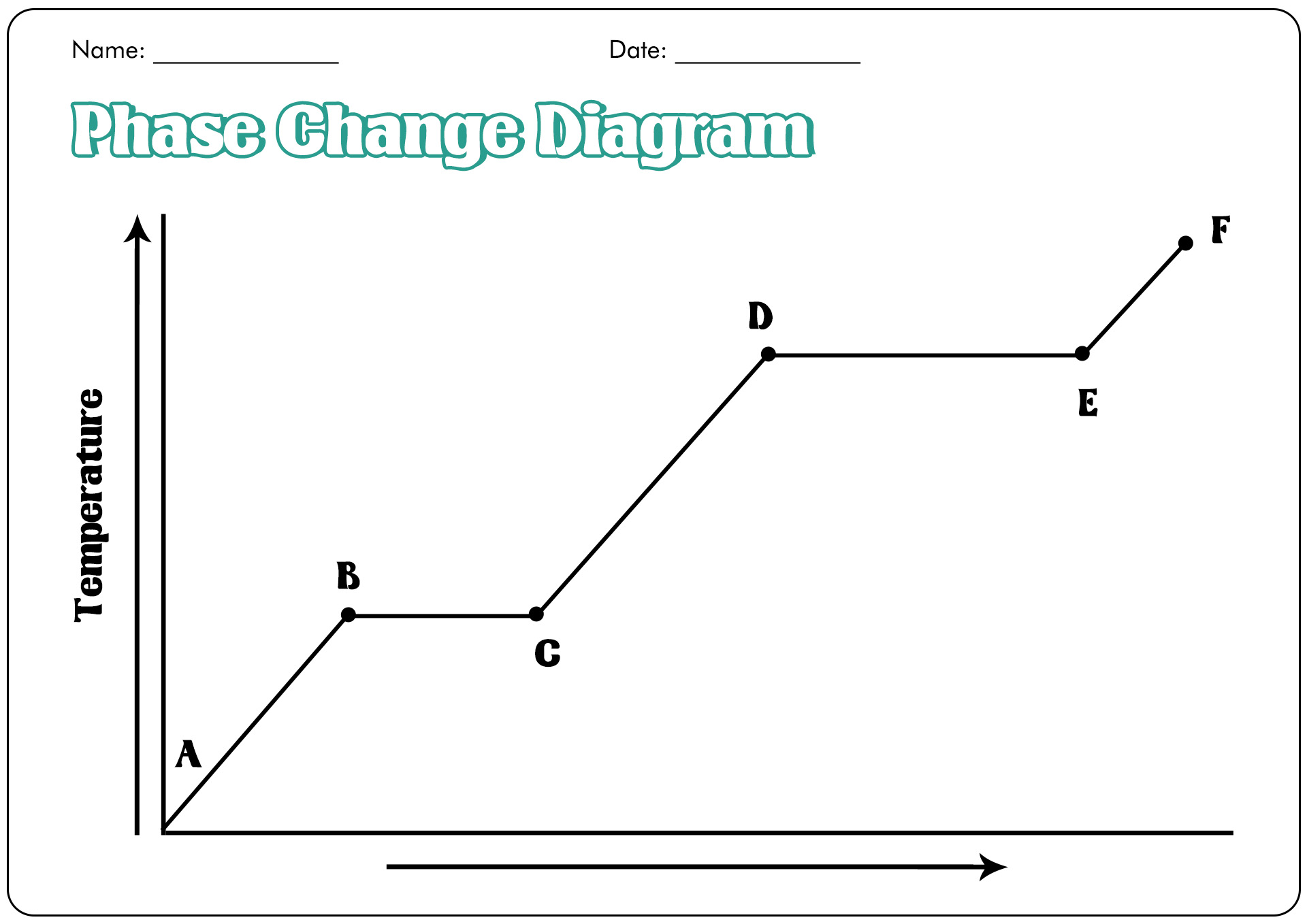
13 Best Images Of Phase Change Worksheet Middle School Blank Phase In the video here, sal uses a horizontal line through the phase diagram. but, it doesn't have to be horizontal. imagine a vertical line through this diagram for water, choose 100 degrees c. as long as you are at 100 c, you can change the phase by changing the pressure on the system. Solution: when two substances or objects at different temperatures are brought into contact, heat will flow from the warmer one to the cooler. the amount of heat that flows is given by. q = mcsΔt (16.3.1) (16.3.1) q = m c s Δ t. where q is heat, m is mass, cs is the specific heat, and Δ t is the temperature change. Phase diagrams are plots that show the different phases of a substance across multiple variables, such as temperature and pressure. learn about the features, types, and examples of phase diagrams, and how they relate to states of matter, intermolecular forces, and physical properties. Learn how to use phase diagrams to predict the phase of a substance at different temperatures and pressures. see examples of phase diagrams for water, carbon dioxide, and argon, and understand the critical point and the triple point.
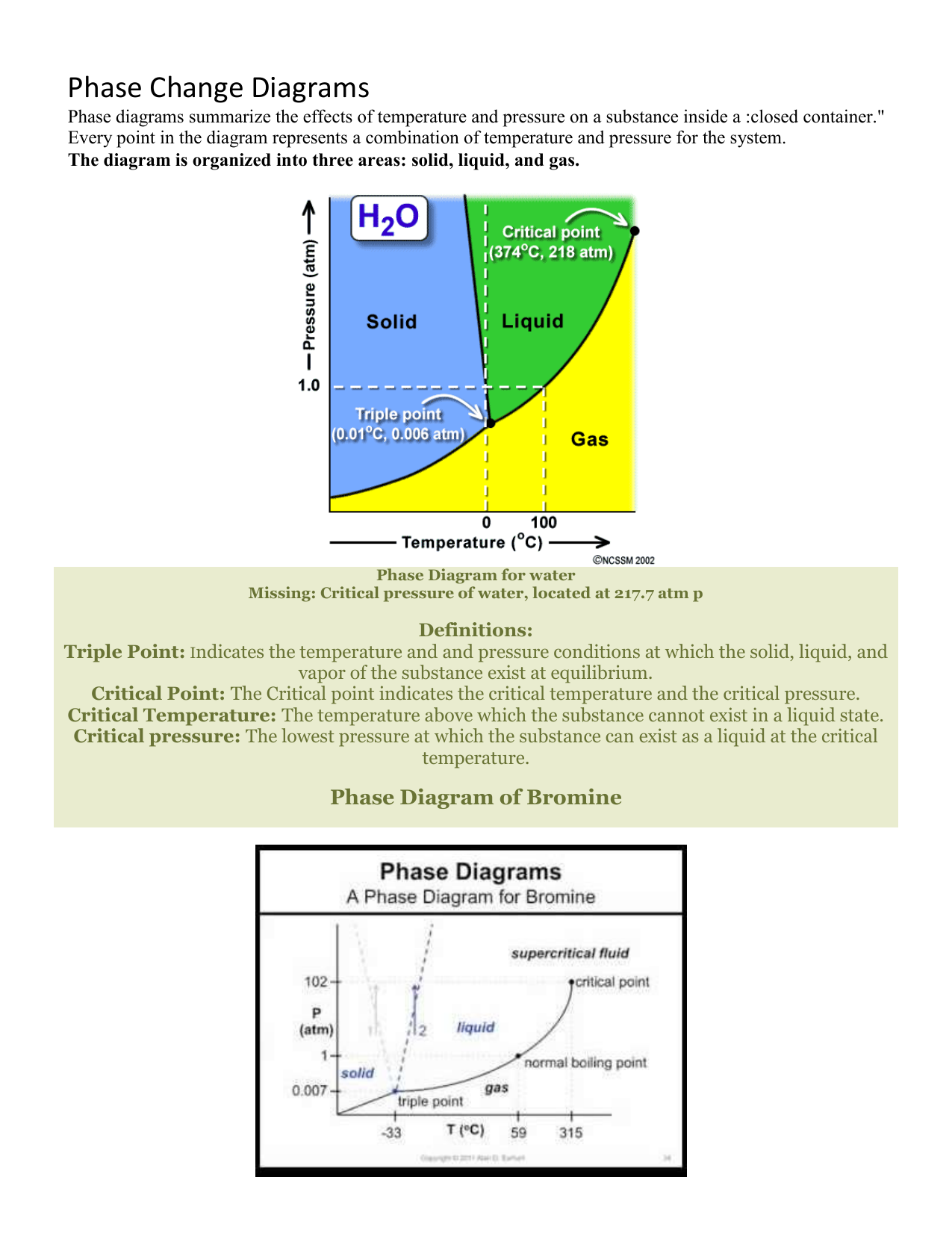
Phase Change Diagrams Phase diagrams are plots that show the different phases of a substance across multiple variables, such as temperature and pressure. learn about the features, types, and examples of phase diagrams, and how they relate to states of matter, intermolecular forces, and physical properties. Learn how to use phase diagrams to predict the phase of a substance at different temperatures and pressures. see examples of phase diagrams for water, carbon dioxide, and argon, and understand the critical point and the triple point. Using the phase diagram for water, we can determine that the state of water at each temperature and pressure given are as follows: (a) solid; (b) liquid; (c) liquid; (d) gas; (e) solid; (f) gas. check your learning what phase changes can water undergo as the temperature changes if the pressure is held at 0.3 kpa? if the pressure is held at 50 kpa?. The phase diagram shows, in pressure–temperature space, the lines of equilibrium or phase boundaries between the three phases of solid, liquid, and gas . the curves on the phase diagram show the points where the free energy (and other derived properties) becomes non analytic: their derivatives with respect to the coordinates (temperature and.
.PNG)
Phase Diagrams Presentation Chemistry Using the phase diagram for water, we can determine that the state of water at each temperature and pressure given are as follows: (a) solid; (b) liquid; (c) liquid; (d) gas; (e) solid; (f) gas. check your learning what phase changes can water undergo as the temperature changes if the pressure is held at 0.3 kpa? if the pressure is held at 50 kpa?. The phase diagram shows, in pressure–temperature space, the lines of equilibrium or phase boundaries between the three phases of solid, liquid, and gas . the curves on the phase diagram show the points where the free energy (and other derived properties) becomes non analytic: their derivatives with respect to the coordinates (temperature and.

Sublimation Phase Diagram

Comments are closed.