Phase Change Diagrams Overview Examples Expii Vrogue Co

What Is Phase Change Explained By Thermal Engineers The region furthest to the left is solids, in the middle is liquids and, the right side is the gas phase. we can develop a phase change diagram for almost any element or molecule. for example, you could look up the phase change diagram for water or carbon dioxide. if you're interested in materials chemistry, they often use phase change diagrams. Remember, the phase boundaries are lines. so they occur at various temperatures and pressures. the molecules' kinetic energies balance the external pressure. the result is that the phase changes are in equilibrium. as we saw, the heating and cooling curves have plateaus. we are rearranging the electrostatic interactions.
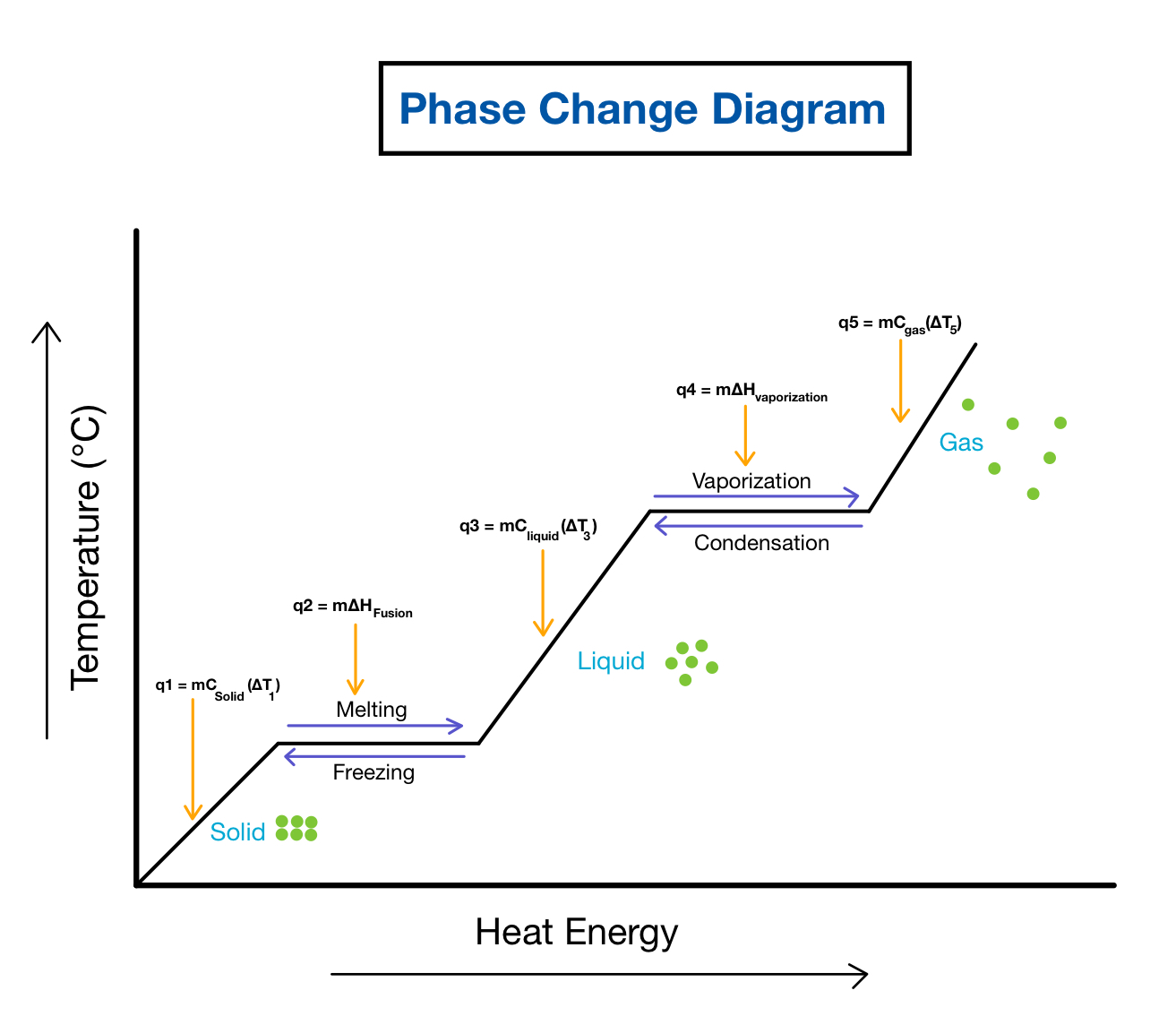
Phase Change Diagrams вђ Overview Examples Expii Phase diagrams for water. water has a unique phase change diagram. the solid liquid boundary has a negative slope. so, it points diagonally left. for most substances, if we apply pressure to a liquid, it freezes. there is more air pushing down which, causes the liquid molecules to get rammed together. Figure 16.3.1 16.3. 1: a heating curve for water. this plot of temperature shows what happens to a 75 g sample of ice initially at 1 atm and −23°c as heat is added at a constant rate: a–b: heating solid ice; b–c: melting ice; c–d: heating liquid water; d–e: vaporizing water; e–f: heating steam. thus the temperature of a system does. A typical phase diagram for a pure substance is shown in figure 3.4.1 3.4. 1. figure 3.4.1 3.4. 1: the physical state of a substance and its phase transition temperatures are represented graphically in a phase diagram. a graph is shown where the x axis is labeled “temperature” and the y axis is labeled “pressure.”. Phase changes. phase changes are physical changes that take place when matter changes energy states, but chemical bonds are not broken or formed. for example, the physical properties of ice, liquid water, and steam are quite different even though they are all \ce {h2o} hx 2o and there is no difference in the molecular structure of the substances.

Phase Change Diagram Of Water Overview Importance Exp Vrogue Co A typical phase diagram for a pure substance is shown in figure 3.4.1 3.4. 1. figure 3.4.1 3.4. 1: the physical state of a substance and its phase transition temperatures are represented graphically in a phase diagram. a graph is shown where the x axis is labeled “temperature” and the y axis is labeled “pressure.”. Phase changes. phase changes are physical changes that take place when matter changes energy states, but chemical bonds are not broken or formed. for example, the physical properties of ice, liquid water, and steam are quite different even though they are all \ce {h2o} hx 2o and there is no difference in the molecular structure of the substances. A phase change or phase transition is a change between solid, liquid, gaseous, and sometimes plasma states of matter. the states of matter differ in the organization of particles and their energy. the main factors that cause phase changes are changes in temperature and pressure. at the phase transition, such as the boiling point between liquid. A phase change is the physical conversion of a substance from one state of matter to another. some examples include melting, freezing, and condensation. phase changes.

Comments are closed.