Phase Change Energy Education

What Is Phase Change Explained By Thermal Engineers A phase change is when matter changes to from one state (solid, liquid, gas, plasma) to another. (see figure 1). these changes occur when sufficient energy is supplied to the system (or a sufficient amount is lost), and also occur when the pressure on the system is changed. the temperatures and pressures under which these changes happen differ. Table 11.3 latent heats of fusion and vaporization, along with melting and boiling points. let’s consider the example of adding heat to ice to examine its transitions through all three phases—solid to liquid to gas. a phase diagram indicating the temperature changes of water as energy is added is shown in figure 11.10.
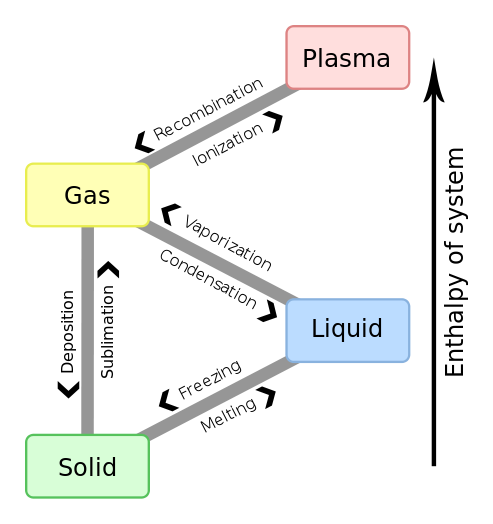
Gas To Liquid Is Called Donnasripratt Latent heat is the heat required for an object to change phase (melt, boil, freeze, etc.). this energy is closely related to enthalpy. [1] in figure 1, very cold ice has heat added to it. the temperature goes up, so that's sensible heat, but once it starts melting, that heat is latent heat (and is represented by the flat parts of the line. At 100oc 100 o c, the water begins to boil and the temperature again remains constant while the water absorbs 539 cal g of heat during this phase change. when all the liquid has become steam vapor, the temperature rises again, absorbing heat at a rate of 0.482cal g ⋅o c 0.482 c a l g ⋅ o c. figure 14.3.3 14.3. 3. The heat q required to change the phase of a sample of mass m is given by. q = mlf q = m l f (melting or freezing) q = mlv q = m l v (evaporating or condensing) where the latent heat of fusion, lf, and latent heat of vaporization, lv, are material constants that are determined experimentally. phase transitions: (a) energy is required to. Sublimation. figure 1: a piece of solid carbon dioxide (dry ice) sublimating from a solid directly into a gas. [1] sublimation is a type of phase change that takes place when a solid turns directly into a gas, skipping the liquid phase. the opposite of sublimation is vapour deposition. the term "sublimation" only applies to a physical change of.
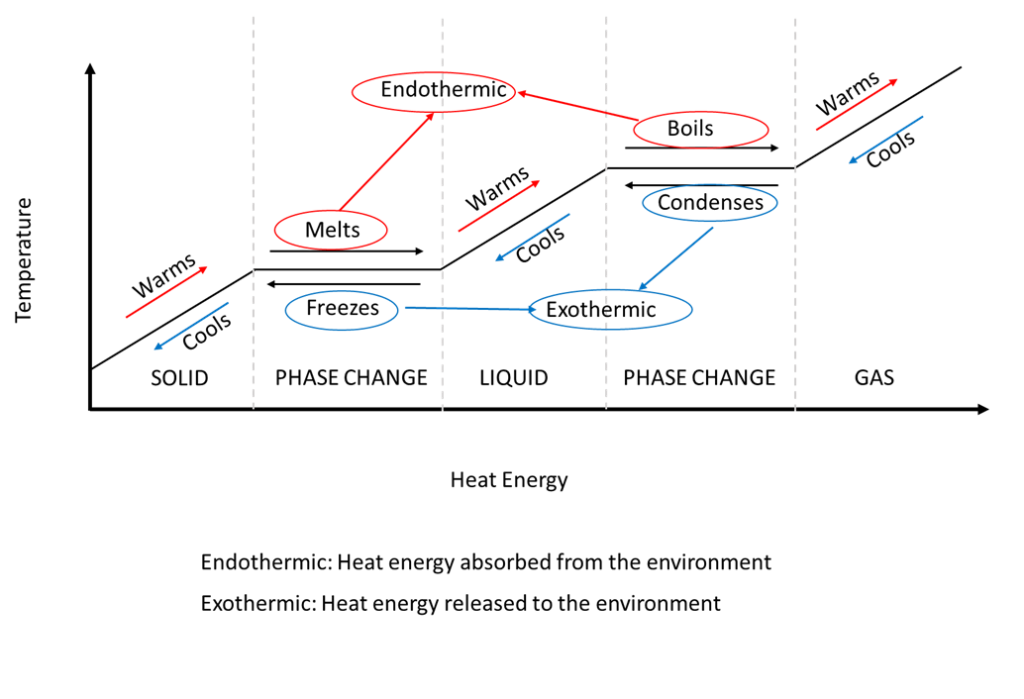
Phase Changes вђ Basic Hvac The heat q required to change the phase of a sample of mass m is given by. q = mlf q = m l f (melting or freezing) q = mlv q = m l v (evaporating or condensing) where the latent heat of fusion, lf, and latent heat of vaporization, lv, are material constants that are determined experimentally. phase transitions: (a) energy is required to. Sublimation. figure 1: a piece of solid carbon dioxide (dry ice) sublimating from a solid directly into a gas. [1] sublimation is a type of phase change that takes place when a solid turns directly into a gas, skipping the liquid phase. the opposite of sublimation is vapour deposition. the term "sublimation" only applies to a physical change of. They are latent, or hidden, because in phase changes, energy enters or leaves a system without causing a temperature change in the system; so, in effect, the energy is hidden. table 1 lists representative values of l f and l v, together with melting and boiling points. the table shows that significant amounts of energy are involved in phase. Phase changes transitions between solid, liquid, and gaseous phases typically involve large amounts of energy compared to the specific heat.if heat were added at a constant rate to a mass of ice to take it through its phase changes to liquid water and then to steam, the energies required to accomplish the phase changes (called the latent heat of fusion and latent heat of vaporization) would.

Phase Change Diagrams вђ Overview Examples Expii They are latent, or hidden, because in phase changes, energy enters or leaves a system without causing a temperature change in the system; so, in effect, the energy is hidden. table 1 lists representative values of l f and l v, together with melting and boiling points. the table shows that significant amounts of energy are involved in phase. Phase changes transitions between solid, liquid, and gaseous phases typically involve large amounts of energy compared to the specific heat.if heat were added at a constant rate to a mass of ice to take it through its phase changes to liquid water and then to steam, the energies required to accomplish the phase changes (called the latent heat of fusion and latent heat of vaporization) would.

Comments are closed.