Phase Diagram Of Sulfur Explanation Phase Diagram Of Sulfur The Phase
Phase Diagram Of Sulfur Explanation Phase Diagram Of Sulfur The Phase Liquid phase: when heated above its melting point of 115.21°c, sulfur transitions into a liquid phase. in this phase, sulfur exhibits a dark amber color and has a higher density compared to its solid form. the liquid phase of sulfur is less stable than the solid phase and can be easily affected by changes in temperature and pressure conditions. 3. – the phase diagram of the sulphur system has four areas or regions. – these are labelled as rhombic sulphur, monoclinic sulphur, liquid sulphur and vapour. – these represent single phase systems which have two degrees of freedom, f = c – p 2 = 1 – 1 2 = 2 – that is, each of the systems s r, s m, s l, and s v are bivariant.

Phase Diagram Of Sulfur Understanding the phase diagram of sulfur is important in determining these conditions. at atmospheric pressure, sulfur melts at 115.21°c and boils at 444.6°c, transitioning from solid to liquid and then to gas. however, the vapor phase of sulfur can also be achieved under different pressure conditions. Explanation: a phase diagram is a chart that shows the conditions of pressure and temperature at which distinct phases occur and coexist at equilibrium. the lines on a phase diagram divide into regions – solid, liquid, and gas. the phase diagram of sulfur is. the diagram is complicated by the fact that sulfur can exist in two crystalline. Low pressure phase diagram of sulfur (schematic). legend: sr, sm, ℓ, and g are respectively the rhombic solid, monoclinic solid, liquid and gaseous phases; t1, t2 and t3 are triple points; c is the critical point. a and b are generic points on the sublimation curve and on the melting line of rhombic sulfur, respectively. Sulfur exhibits a very complicated phase behavior that has puzzled chemists for over a century; what you see here is the greatly simplified phase diagram shown in most textbooks. the difficulty arises from the tendency of s 8 molecules to break up into chains (especially in the liquid above 159°c) or to rearrange into rings of various sizes (s.

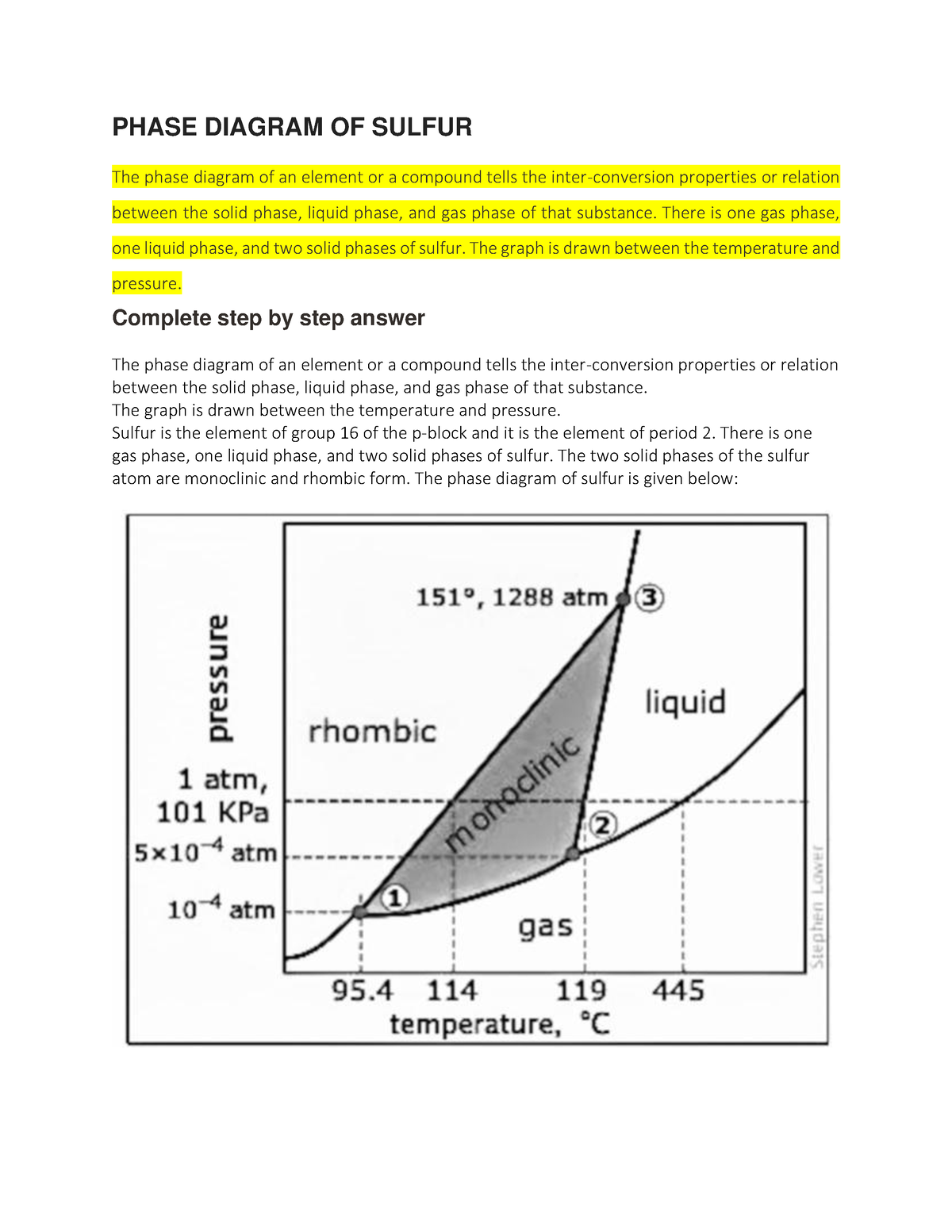
Use The Phase Diagram For Sulfur For Question 75 The Solid Forms Of Low pressure phase diagram of sulfur (schematic). legend: sr, sm, ℓ, and g are respectively the rhombic solid, monoclinic solid, liquid and gaseous phases; t1, t2 and t3 are triple points; c is the critical point. a and b are generic points on the sublimation curve and on the melting line of rhombic sulfur, respectively. Sulfur exhibits a very complicated phase behavior that has puzzled chemists for over a century; what you see here is the greatly simplified phase diagram shown in most textbooks. the difficulty arises from the tendency of s 8 molecules to break up into chains (especially in the liquid above 159°c) or to rearrange into rings of various sizes (s. The phase diagram for sulfur is shown here. the rhombic and monoclinic states are two solid states with different structures. b. which of the two solid states of sulfur is more dense?. There is one gas phase, one liquid phase, and two solid phases of sulfur. the two solid phases of the sulfur atom are monoclinic and rhombic form. the phase diagram of sulfur is given below: there are three triple points of sulfur and it is indicated in the diagram with numbers 1, 2, and 3.

Comments are closed.