Phase Diagrams Simplified

Phase Diagram Definition Explanation And Diagram In the video here, sal uses a horizontal line through the phase diagram. but, it doesn't have to be horizontal. imagine a vertical line through this diagram for water, choose 100 degrees c. as long as you are at 100 c, you can change the phase by changing the pressure on the system. Fig. 5. another very interesting condition can be observed from the phase diagram of water i.e., if we decrease the pressure of liquid phase of water while keeping the temperature constant at a certain value, the line of lower pressure crosses the liquid phase space then solid phase space and finally ended in vapor phase space (fig. 6).
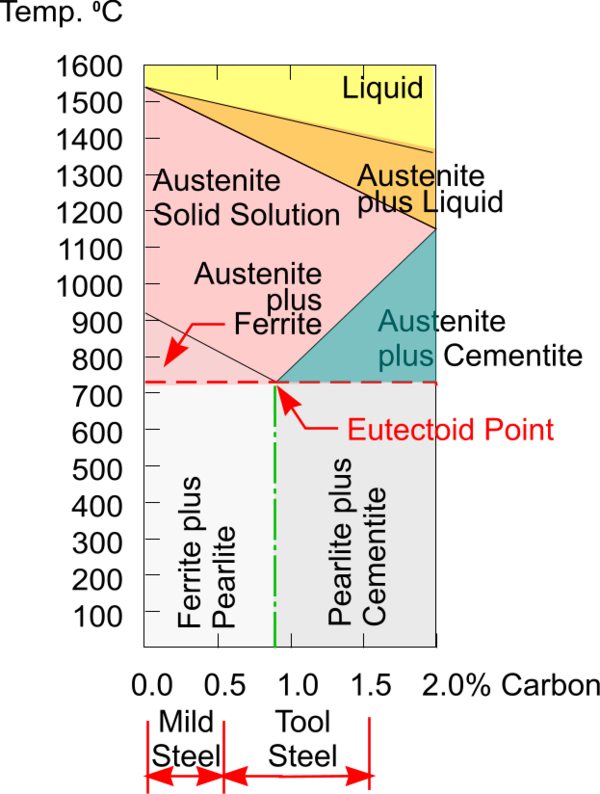
Phase Diagrams Dt Online Webpage kentchemistry links matter phasediagram.htmthis short video dissects a phase diagrams and explains how to use it to determine a phase. Phase diagram. a phase diagram represents the various physical states or phases of matter at different pressures and temperatures. in other words, it summarizes the effect of pressure and temperature on the nature of a substance. a phase diagram is divided into three areas representing the substances’ solid, liquid, and gaseous phases [1 4]. A phase diagram in physical chemistry, engineering, mineralogy, and materials science is a type of chart used to show conditions (pressure, temperature, etc.) at which thermodynamically distinct phases (such as solid, liquid or gaseous states) occur and coexist at equilibrium . Figure chapter4.7: eutectic phase diagram. the binary eutectic phase diagram has several distinctive features one being a solid solid phase mixture, limit of solubility at different temperatures, and an invariant point in the phase diagram, the eutectic point. the solubility limit is the maximum amount of solute that you can integrate into the.

Phase Diagrams Chemtalk A phase diagram in physical chemistry, engineering, mineralogy, and materials science is a type of chart used to show conditions (pressure, temperature, etc.) at which thermodynamically distinct phases (such as solid, liquid or gaseous states) occur and coexist at equilibrium . Figure chapter4.7: eutectic phase diagram. the binary eutectic phase diagram has several distinctive features one being a solid solid phase mixture, limit of solubility at different temperatures, and an invariant point in the phase diagram, the eutectic point. the solubility limit is the maximum amount of solute that you can integrate into the. Phase diagram is a graphical representation of the physical states of a substance under different conditions of temperature and pressure. a typical phase diagram has pressure on the y axis and temperature on the x axis. as we cross the lines or curves on the phase diagram, a phase change occurs. in addition, two states of the substance coexist. One component phase diagram. figure 1 illustrates the temperatures and pressures at which water can exist as a solid, liquid or vapor. the curves represent the points at which two of the phases coexist in equilibrium. at the point tt vapor, liquid and solid coexist in equilibrium. in the fields of the diagram (phase fields) only one phase exists.

Phase Diagrams Youtube Phase diagram is a graphical representation of the physical states of a substance under different conditions of temperature and pressure. a typical phase diagram has pressure on the y axis and temperature on the x axis. as we cross the lines or curves on the phase diagram, a phase change occurs. in addition, two states of the substance coexist. One component phase diagram. figure 1 illustrates the temperatures and pressures at which water can exist as a solid, liquid or vapor. the curves represent the points at which two of the phases coexist in equilibrium. at the point tt vapor, liquid and solid coexist in equilibrium. in the fields of the diagram (phase fields) only one phase exists.

Comments are closed.