Phases Of Drugs
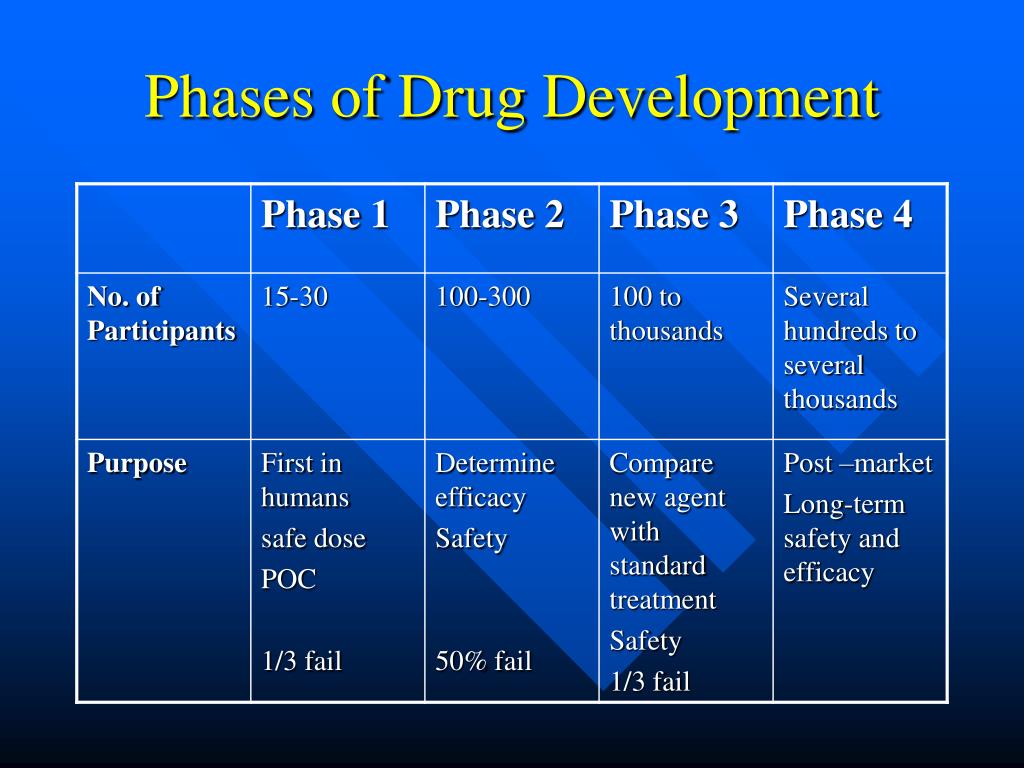
Phases Of Drugs Phase 0 of a clinical trial is done with a very small number of people, usually fewer than 15. investigators use a very small dose of medication to make sure it isn’t harmful to humans before. Step 1 discovery and development. discovery and development research for a new drug begins in the laboratory. more information. step 2 preclinical research. preclinical research drugs undergo.
From Drug Use To Drug Abuse The Stages Of Addiction During phase 1 studies, researchers test a new drug in normal volunteers (healthy people). in most cases, 20 to 80 healthy volunteers or people with the disease condition participate in phase 1. What are the 4 phases of drug approval? if the fda gives the green light, the investigational drug will enter several phases of clinical trials and post marketing approval: phase 1: phase 1 focuses on safety. about 20 to 80 healthy volunteers are recruited to establish a drug's safety and side effects and takes about 1 year. The phases of clinical research are the stages in which scientists conduct experiments with a health intervention to obtain sufficient evidence for a process considered effective as a medical treatment. [ 1] for drug development, the clinical phases start with testing for drug safety in a few human subjects, then expand to many study. In fact, the overall probability of success for new molecular entities is only 12%. 2. to be deemed a “success,” a new drug must make it through five specific phases: 1) discovery and development, 2) preclinical research, 3) clinical research, 4) fda review, and 5) safety monitoring. below, we explore each step in more detail.

Pin On Notes The phases of clinical research are the stages in which scientists conduct experiments with a health intervention to obtain sufficient evidence for a process considered effective as a medical treatment. [ 1] for drug development, the clinical phases start with testing for drug safety in a few human subjects, then expand to many study. In fact, the overall probability of success for new molecular entities is only 12%. 2. to be deemed a “success,” a new drug must make it through five specific phases: 1) discovery and development, 2) preclinical research, 3) clinical research, 4) fda review, and 5) safety monitoring. below, we explore each step in more detail. Drug development is the process of bringing a new pharmaceutical drug to the market once a lead compound has been identified through the process of drug discovery.it includes preclinical research on microorganisms and animals, filing for regulatory status, such as via the united states food and drug administration for an investigational new drug to initiate clinical trials on humans, and may. During this phase, effort is focussed on identifying a lead chemical structure, designing, testing and fine tuning it and ensure that it meets all the criteria required for development into a drug product. an overview of the main stages that constitute a typical drug discovery project, from the point of identification of a target to the.

Comments are closed.