Physical Properties Of Sulphur Health Checklist

Physical Properties Of Sulphur Health Checklist Physical properties of sulfur. sulfur has an atomic weight of 32.066 grams per mole and is part of group 16, the oxygen family. it is a nonmetal and has a specific heat of 0.706 j g 1 o c 1. the electron affinity is 200 kj mol 1 and the electronegativity is 2.58 (unitless). sulfur is typically found as a light yellow, opaque, and brittle solid. Physical properties of sulfur. sulfur is responsible for the formation of many polyatomic molecules. one of the most common types of molecules associated with sulphur is octasulfur. it is odorless and bright yellow, and it exists as a soft solid. the melting point of sulphur is approximately 115.21° c, and its boiling point is approximately.

Physical Properties Of Sulphur Health Checklist The frasch process, illustrated in figure 18.59, is important in the mining of free sulfur from enormous underground deposits in texas and louisiana.superheated water (170 °c and 10 atm pressure) is forced down the outermost of three concentric pipes to the underground deposit. Atomic structure of sulfur. properties of sulfur. sulfur, an essential element known for its bright yellow crystals and distinct smell, is a non metal and the tenth most abundant element in the universe. this guide delves deep into the physical and chemical properties of sulfur, offering educators and students a comprehensive understanding. Chemical characteristics. sulfur is a reactive metal. it forms compounds with all other elements, except nitrogen, gold, iodine, platinum and the nobel gases. upon combustion, sulfur gives out a blue flame and produces sulfur oxide that has pungent odor. sulfur have various oxidation states, 2, 4 , 6 and 6 and 4 are more common [3]. Vulcanization is the process of adding sulfur to rubber to make it stiff and hard. sulfuric acid is also used in smaller amounts to make explosives, water treatment chemicals, storage batteries, pesticides, reaction of sulfuric acid and sugar. drugs, synthetic fibers, and many other chemicals used in everyday life.

Physical Properties Of Sulphur Chemical characteristics. sulfur is a reactive metal. it forms compounds with all other elements, except nitrogen, gold, iodine, platinum and the nobel gases. upon combustion, sulfur gives out a blue flame and produces sulfur oxide that has pungent odor. sulfur have various oxidation states, 2, 4 , 6 and 6 and 4 are more common [3]. Vulcanization is the process of adding sulfur to rubber to make it stiff and hard. sulfuric acid is also used in smaller amounts to make explosives, water treatment chemicals, storage batteries, pesticides, reaction of sulfuric acid and sugar. drugs, synthetic fibers, and many other chemicals used in everyday life. Material and physical properties. melting point 115.21 °c enthalpy of fusion (molar) 1.727 kj mol 1 boiling point 444.61 °c enthalpy of vaporization 9.62 kj mol 1 density 2.07 g cm 3 (α s). 1. sulfur is a constituent of many proteins and is essential for life. figure 18.10.1 18.10. 1: volcanic gases contain hydrogen sulfide. (credit: daniel julie wikimedia commons) the frasch process, illustrated in figure 18.10.2 18.10. 2, is important in the mining of free sulfur from enormous underground deposits in texas and louisiana.
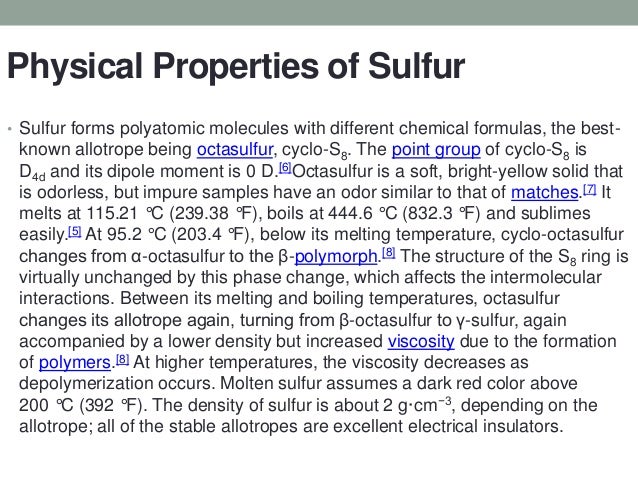
Physical Properties Of Sulphur Material and physical properties. melting point 115.21 °c enthalpy of fusion (molar) 1.727 kj mol 1 boiling point 444.61 °c enthalpy of vaporization 9.62 kj mol 1 density 2.07 g cm 3 (α s). 1. sulfur is a constituent of many proteins and is essential for life. figure 18.10.1 18.10. 1: volcanic gases contain hydrogen sulfide. (credit: daniel julie wikimedia commons) the frasch process, illustrated in figure 18.10.2 18.10. 2, is important in the mining of free sulfur from enormous underground deposits in texas and louisiana.

Comments are closed.