Ppt Acids And Bases Powerpoint Presentation Free Download Id 4503088

Ppt Acids And Bases Powerpoint Presentation Free Download Id 4503088 Acids and bases (courtesy of l. scheffler, lincoln high school, 2010) acids • react with certain metals to produce hydrogen gas. • react with carbonates and bicarbonates to produce carbon dioxide gas bases • have a bitter taste • feel slippery. • many soaps contain bases. properties of acids • produce h (as h3o ) ions in water (the. Determine the ph of salt solution. polyprotic weak acids calculate the ph and the concentration of other species solution of weak polyprotic acid. 3 the acid base concept acids and bases are chemical “opposites” they react to neutralize each other at the heart of the reaction is a “tug of war” for protons or h. .

Ppt Acids And Bases Powerpoint Presentation Free Download Id 4503088 Acids and bases. acids and bases. acids:have a sour tasteeats away materialsalways wear safety glasses when using acide.g. sulphuric acid, citric acid. acids and bases. indicator: this will indicate whether a substance is acidic.e.g. litmus is a type of indicator.acid turns litmus from blue to redbases turns. 401 views • 9 slides. Acids and bases: introduction. section 19.1. objectives. identify the physical and chemical properties of acids and bases classify solutions as acidic, basic, or neutral compare the arrhenius and bronsted lowry models of acids and bases. key terms. acidic solutions basic solutions. download presentation. acid. An acid is a substance that dissociates in water to produce h ions. a base is a substance that dissociates in water to produce oh ions. a strong acid almost completely dissociates in water to give h ions. . a weak acid slightly dissociates in water to give h ions. a strong base almost completely dissociates in water to give oh ions. 1) acids release h ions in water and bases release oh ions. strong acids and bases dissociate completely while weak acids and bases only partially dissociate. 2) acids taste sour, turn litmus red, and react with metals and carbonates. bases taste bitter and feel slippery. 3) the ph scale measures acidity and alkalinity, with values below 7.
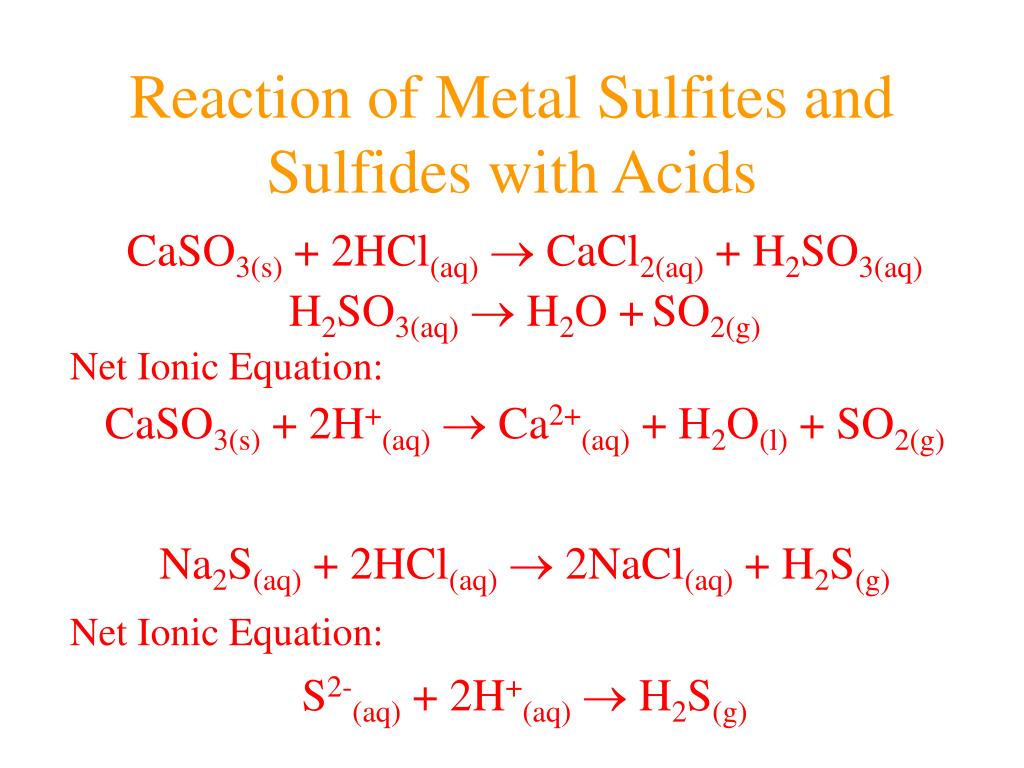
Ppt Acids And Bases Powerpoint Presentation Free Download Id 4503088 An acid is a substance that dissociates in water to produce h ions. a base is a substance that dissociates in water to produce oh ions. a strong acid almost completely dissociates in water to give h ions. . a weak acid slightly dissociates in water to give h ions. a strong base almost completely dissociates in water to give oh ions. 1) acids release h ions in water and bases release oh ions. strong acids and bases dissociate completely while weak acids and bases only partially dissociate. 2) acids taste sour, turn litmus red, and react with metals and carbonates. bases taste bitter and feel slippery. 3) the ph scale measures acidity and alkalinity, with values below 7. Bitter taste, soapy feel. high concentration of oh ions. conduct electricity. good conductor strong base. weak conductor weak base. react with acids to form water and a salt. ph of 8 14, the higher the ph the stronger the base. example baking soda. what are some tests you could do to figure out. Biologica.edu. this document defines acids and bases and discusses their properties. it states that acids produce hydrogen ions in water and have a ph less than 7, while bases are metal oxides or hydroxides that react with acids to form salts and water. common acids include hydrochloric acid and sulfuric acid, while common bases include sodium.
Naming Acids And Bases Ppt Bitter taste, soapy feel. high concentration of oh ions. conduct electricity. good conductor strong base. weak conductor weak base. react with acids to form water and a salt. ph of 8 14, the higher the ph the stronger the base. example baking soda. what are some tests you could do to figure out. Biologica.edu. this document defines acids and bases and discusses their properties. it states that acids produce hydrogen ions in water and have a ph less than 7, while bases are metal oxides or hydroxides that react with acids to form salts and water. common acids include hydrochloric acid and sulfuric acid, while common bases include sodium.
Ppt Acids And Bases Powerpoint Presentation Free Download

Comments are closed.