Ppt Chapter 24 Transition Metals Coordination Compounds Powerpointо
Ppt Chapter 24 Transition Metals And Coordination Compoundsођ Geometries anne marie helmenstine, ph.d., about guide. chapter 24 transition metals & coordination compounds. 24.2 properties of transition metals review electron configuration trends in the periodic table 24.3 coordination compounds the basics example of naming 24.4 structure and isomerization. slideshow 2330059 by ishana. Chemistry: a molecular approach , 1 st ed. nivaldo tro. chapter 24 transition metals and coordination compounds. roy kennedy massachusetts bay community college wellesley hills, ma. 2007, prentice hall. gemstones.
Ppt Chapter 24 Transition Metals Coordination Compounds ођ Transition metals contain e in d orbitals breath – slow down! 16 a little background in 1893, swiss chemist alfred werner came up with the idea that a central metal could have 2 types of interactions primary valence – oxidation state of the central metal secondary valence – number of molecules or ions directly attached to the central metal or coordination number example: [co(nh3)6]cl3. Presentation transcript. chemistry: a molecular approach, 2nd ed.nivaldo tro chapter 24transition metals and coordination compounds roy kennedy massachusetts bay community college wellesley hills, ma. gemstones • the colors of rubies and emeralds are both due to the presence of cr3 ions – the difference lies in the crystal hosting the ion. Tro, chemistry: a molecular approach3 properties and electron configuration of transition metals the properties of the transition metals are similar to each other and very different tot he properties of the main group metals high melting points, high densities, moderate to very hard, and very good electrical conductors in general, the transition metals have two valence electrons – we are. Chapter 24 transition metals and coordination compounds 2007, prentice hall chemistry: a molecular approach, 1 st ed. nivaldo tro roy kennedy massachusetts. 25 1werner’s theory of coordination compounds: an overview.
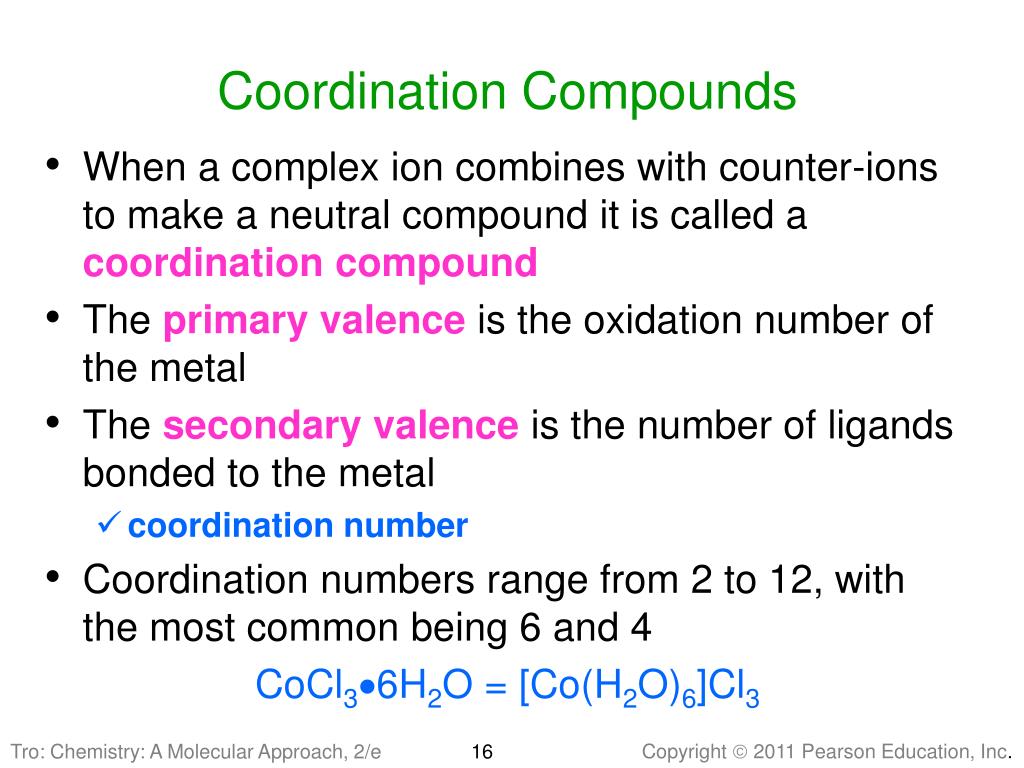
Ppt Chapter 24 Transition Metals And Coordination Compoundsођ Tro, chemistry: a molecular approach3 properties and electron configuration of transition metals the properties of the transition metals are similar to each other and very different tot he properties of the main group metals high melting points, high densities, moderate to very hard, and very good electrical conductors in general, the transition metals have two valence electrons – we are. Chapter 24 transition metals and coordination compounds 2007, prentice hall chemistry: a molecular approach, 1 st ed. nivaldo tro roy kennedy massachusetts. 25 1werner’s theory of coordination compounds: an overview. • suggested in 1893 that metal ions have primary and secondary valences. ! primary valence equals the metal’s oxidation number ! secondary valence is the number of atoms directly bonded to the metal (coordination number) co(iii) oxidation state coordination # is 6 cl. Coordination compounds class 12 1.pptx. this document provides an overview of coordination compounds and key concepts in coordination chemistry. it discusses the discovery of coordination compounds, werner's theory of coordination compounds, types of ligands and isomerism in coordination compounds. specifically, it introduces coordination.

Comments are closed.