Ppt Investigator Responsibilities For Research Powerpoint

Ppt Investigator Responsibilities For Research Powerpoint Investigators responsibilities during clinical trials. jul 22, 2021 • download as pptx, pdf •. 1 like • 601 views. b. bhavyarajan2. this presentation was presented by pruthvi raj, tejaswini, and myself bhavya rajan at clinosol research pvt ltd. Investigator responsibilities for research. shannon simmons, ba, cip march 14, 2014 process improvement team vanderbilt human research protections program. agenda. review investigator responsibilities discuss fda warning letters q & a. pi responsibilities. slideshow 1625613 by mabli.

Ppt Investigator Responsibilities For Research Powerpoint Powerpoint presentation. julie bouma, jacob holloway and cathy peterson. wmed hrpp. investigator responsibilities for clinical trials research. introduction. this comprehensive, educational presentation for clinical trials research is a resource tool provided by wmed homer stryker m.d. school of medicine for researchers involved in sponsor and. Biostatistics roles and responsibilities in clinical research | pubrica. this presentation explains the roles and responsibilities of biostatistics in clinical researchr r biostatistics helps to find answer for research question in biology, medicine and public healthr how a new drug worksr what causes cancerr what is the reason for many diseasesr how long could a person survive with a. 4. the investigator(s) should be qualified by education, training, and experience to assume responsibility for the proper conduct of the trial, should meet all the qualifications specified by the applicable regulatory requirement(s), and should provide evidence of such qualifsications through up to date curriculum vitae and or other relevant documentation requested by the sponsor, the irb iec. Investigator responsibilities for research. jan zolkower, mshl, cip, ccrp january 13, 2012. agenda. review investigator responsibilities discuss recent fda warning letters and ohrp determination letters review of advanced notice of proposed rulemaking (anprm) q & a. pi responsibilities.
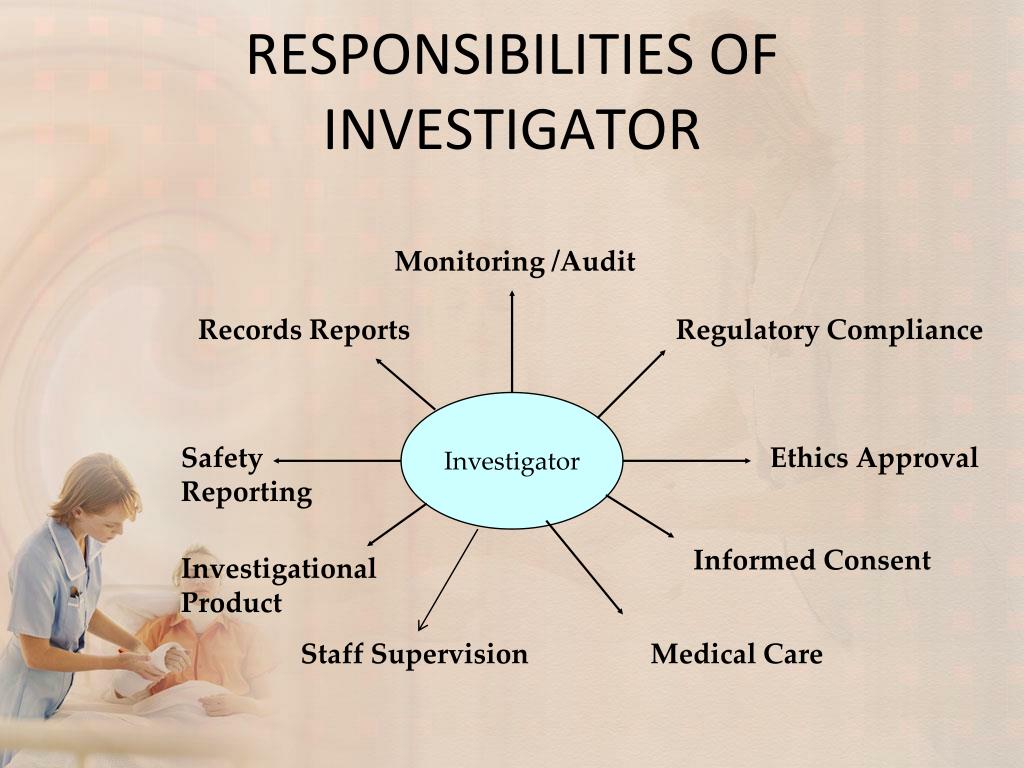
Ppt Responsibilities Of Investigator Powerpoint Presentation Free 4. the investigator(s) should be qualified by education, training, and experience to assume responsibility for the proper conduct of the trial, should meet all the qualifications specified by the applicable regulatory requirement(s), and should provide evidence of such qualifsications through up to date curriculum vitae and or other relevant documentation requested by the sponsor, the irb iec. Investigator responsibilities for research. jan zolkower, mshl, cip, ccrp january 13, 2012. agenda. review investigator responsibilities discuss recent fda warning letters and ohrp determination letters review of advanced notice of proposed rulemaking (anprm) q & a. pi responsibilities. Ich gcp. jun 1, 2018 • download as pptx, pdf •. 134 likes • 44,564 views. suman baishnab. this presentation is a brief overview of ich gcp guidelines. although ich gcp is a very vast topic, still this presentation will cover almost all the points. the reader will be able to discuss about the roles and responsibilities of various personnel. Title: investigator responsibilities in clinical research 1 investigator responsibilities in clinical research . melissa adde ; director, clinical trials office, inctr; inctr.org. 2 topics to be discussed. overview of clinical trials ; good clinical practice ; the research team ; the role and responsibilities of the principal investigator ; 3.

Ppt Investigator Responsibilities For Research Powerpoint Ich gcp. jun 1, 2018 • download as pptx, pdf •. 134 likes • 44,564 views. suman baishnab. this presentation is a brief overview of ich gcp guidelines. although ich gcp is a very vast topic, still this presentation will cover almost all the points. the reader will be able to discuss about the roles and responsibilities of various personnel. Title: investigator responsibilities in clinical research 1 investigator responsibilities in clinical research . melissa adde ; director, clinical trials office, inctr; inctr.org. 2 topics to be discussed. overview of clinical trials ; good clinical practice ; the research team ; the role and responsibilities of the principal investigator ; 3.

Comments are closed.