Ppt Transition Metal Chemistry And Coordination Compounds Pow

Ppt Transition Metal Chemistry And Coordination Compounds Powerp Faculty. steven dessens. notes and practice problems. chem 1412 general chemistry ii (with lab) chem 1412 previous textbook powerpoints. chem 1412 chang powerpoints. chapter 22 transition metal chemistry and coordination compounds. 19 transition metal chemistry and coordination compounds lecture 21 22 20 crystal field theory model explaining bonding for transition metal complexes • originally developed to explain properties for crystalline material • basic idea: electrostatic interaction between lone pair electrons result in coordination.

Ppt Chapter 24 Transition Metals Coordination Compounds Powerpoint Feb 01, 2014. 830 likes | 1.16k views. transition metals and coordination chemistry. 21.1 the transition metals: a survey 21.2 the first row transition metals 21.3 coordination compounds 21.4 isomerism 21.5 bonding in complex ions: the localized electron model 21.6 the crystal field model. download presentation. The transition metals 22.1. 13 naming coordination compounds the cation is named before the anion. within a complex ion, the ligands are named first in alphabetical order and the metal atom is named last. Transition metals. this document provides information about transition metals and their coordination compounds. it discusses the key characteristics of transition metals, including having multiple oxidation states and forming colored compounds. transition metals form coordination complexes by acting as lewis acids and coordinating with ligands. Ionic compounds with transition metals most compounds are colored because the transition metal ion in the complex ion can absorb visible light of specific wavelengths. many compounds are paramagnetic. 7. electron configurations example v: [ar]4s23d3 fe: [ar]4s23d6 exceptions: cr and cu cr: [ar]4s13d5 cu: [ar]4s13d10. 8.
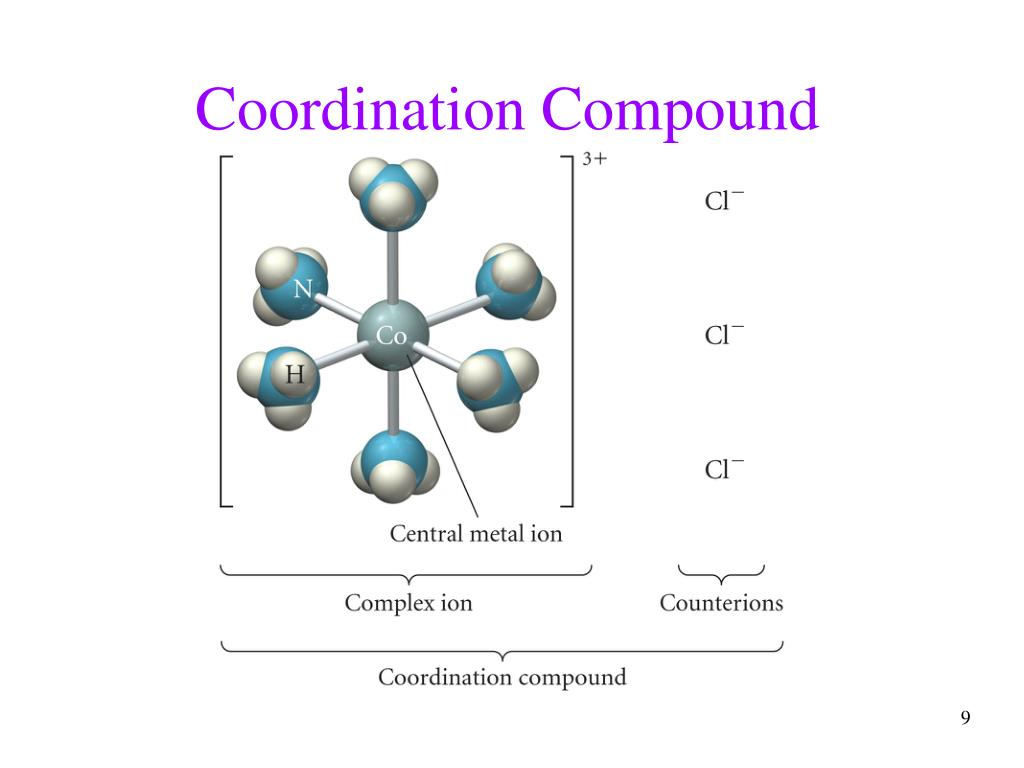
Ppt Transition Metals And Coordination Compounds Powerpoint Transition metals. this document provides information about transition metals and their coordination compounds. it discusses the key characteristics of transition metals, including having multiple oxidation states and forming colored compounds. transition metals form coordination complexes by acting as lewis acids and coordinating with ligands. Ionic compounds with transition metals most compounds are colored because the transition metal ion in the complex ion can absorb visible light of specific wavelengths. many compounds are paramagnetic. 7. electron configurations example v: [ar]4s23d3 fe: [ar]4s23d6 exceptions: cr and cu cr: [ar]4s13d5 cu: [ar]4s13d10. 8. Overview of transition metal complexes. the coordinate covalent or dative bond applies in l: m. lewis bases are called ligands—all serve as ‐donors some are ‐donors as well, and some are ‐acceptors. specific coordination number and geometries depend on metal and number of d‐electrons. Chemistry of coordination compounds. chemistry of coordination compounds. chapter 24. general remarks. a coordination complex or metal complex, consists of an atom or ion (usually metallic), and a surrounding array of bound molecules or anions, that are in turn known as ligands or complex agents . 11.13k views • 62 slides.

Comments are closed.