Ppt Types Of Primary Chemical Bonds Powerpoint Presentation Free
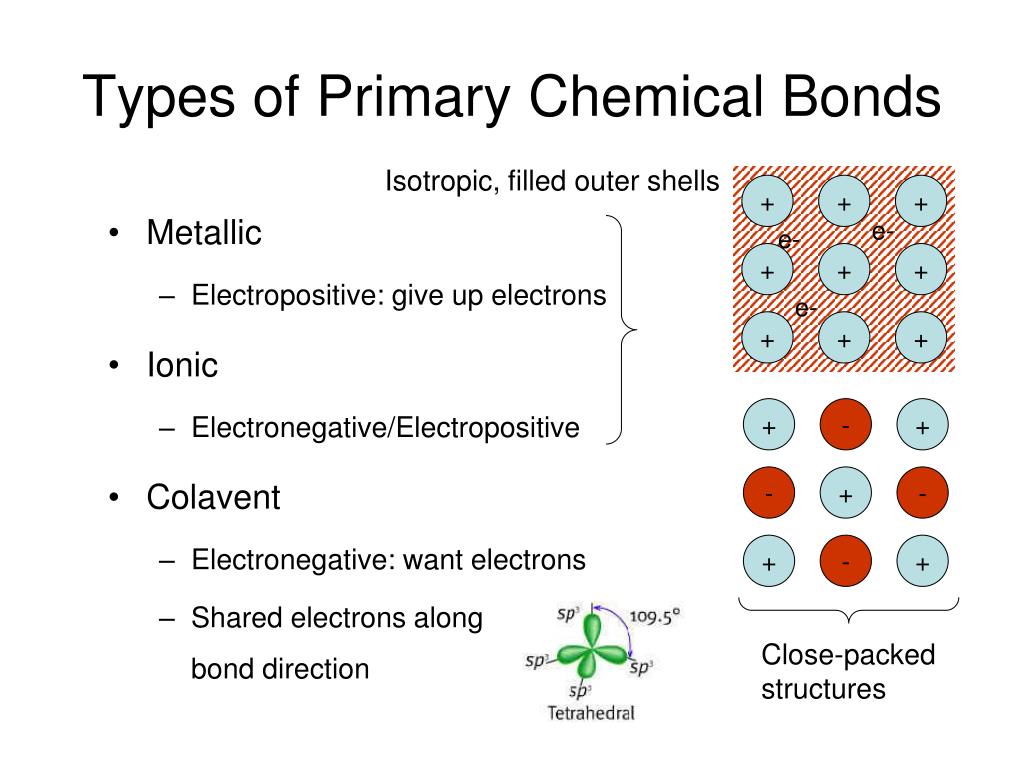
Ppt Types Of Primary Chemical Bonds Powerpoint Presentation Free Ionic bonding: summary. an ionic bond is the electrostatic attraction formed between ( ) cations and ( ) anions. the cations and anions are formed when a non metal (high en) steals an electron from a metal (low ie). this transfer of electrons gives each atom a filled valence shell (stable octet) of electrons. J. jerome bigael. this document discusses different types of chemical bonds including covalent bonds, ionic bonds, metallic bonds, and hydrogen bonds. it explains that a chemical bond is an attraction between atoms that allows the formation of compounds. covalent bonds involve the sharing of electron pairs between atoms, while ionic bonds.

Ppt Types Of Chemical Bonds Powerpoint Presentation Free D Types of chemical bonds. types of chemical bonds. bond : force that holds groups of two or more atoms together and makes the atoms function as a unit. example: h o h bond energy : energy required to break a bond. ionic bond : attractions between oppositely charged ions. 951 views • 37 slides. Atoms tend to gain, lose, or share electrons in order to get a full set of valence electrons. “octet” – most atoms need 8 valence electrons for a full set. gaining or losing → ions = ionic bonding. sharing = covalent bonding. “dogs teaching chemistry”. Steven dessens. notes and practice problems. chem 1411 general chemistry i (with lab) chem 1411 powerpoints from previous textbooks. chem 1411 chang powerpoints. chapter 9 chemical bonding i: basic concepts. Alkenes. 2. methene does not exist because alkenes have a double bond between two carbon atoms – methane would have only one carbon atom. 3. when one carbon atom is added to the chain, the number of hydrogen atoms increases by two. the number of hydrogen atoms is double the number of carbon atoms. 4.

Ppt Types Of Primary Chemical Bonds Powerpoint Presentation Free Steven dessens. notes and practice problems. chem 1411 general chemistry i (with lab) chem 1411 powerpoints from previous textbooks. chem 1411 chang powerpoints. chapter 9 chemical bonding i: basic concepts. Alkenes. 2. methene does not exist because alkenes have a double bond between two carbon atoms – methane would have only one carbon atom. 3. when one carbon atom is added to the chain, the number of hydrogen atoms increases by two. the number of hydrogen atoms is double the number of carbon atoms. 4. Chemical bonds. this document discusses different types of chemical bonds including ionic bonding, covalent bonding, and metallic bonding. ionic bonding involves the electrostatic attraction between oppositely charged ions when atoms gain or lose electrons. covalent bonding occurs when atoms share pairs of electrons to gain stability. This document discusses the four main types of chemical bonds: ionic bonds, covalent bonds, hydrogen bonds, and metallic bonds. ionic bonds involve the transfer of electrons between atoms. covalent bonds involve the sharing of electrons between two atoms. hydrogen bonds are electrostatic attractions between hydrogen atoms covalently bonded to.

Comments are closed.