Preparation Properties Of Sodium Sulfide

Preparation Properties Of Sodium Sulfide Youtube Chemical properties. sodium sulfide has a highly harmful substances that contains the autoignition temperature over 480 c. it is more hazardous to the environment. the melting point is very high and decomposed at the boiling point. it is insoluble in ether and slightly soluble in alcohol. it is antifluorite in structure. Sodium sulfide is a chemical compound with the formula na 2 s, or more commonly its hydrate na 2 s·9h 2 o. both the anhydrous and the hydrated salts in pure crystalline form are colorless solids, although technical grades of sodium sulfide are generally yellow to brick red owing to the presence of polysulfides and commonly supplied as a crystalline mass, in flake form, or as a fused solid.
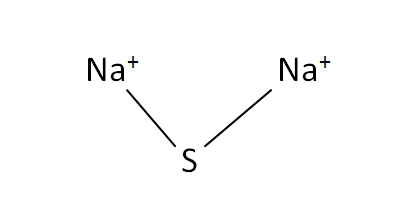
Sodium Sulfide Formula Chemical Properties Preparation Uses Sodium sulfide (na2s) sodium sulfide is an inorganic chemical compound with the formula na2s. it is a colourless water soluble compound. the molecular mass of sodium sulfide is 78.0452 g mol. to learn more about structure, chemical properties, physical properties with uses and faqs of sodium sulfide, visit byju’s for detailed explanations. Properties chemical. sodium sulfide reacts with acids to release hydrogen sulfide. na 2 s 2 hx → h 2 s 2 nax physical. sodium sulfide is a white solid, though impure samples can be yellow or reddish. it has an unpleasant odor of rotten eggs, from the hydrogen sulfide released by the hydrolysis in moist air. availability. Sodium sulfide, or na2s, is an inorganic chemical compound with the formula of na2s that has risen to prominence in the organic chemical industry. it's a powerful alkaline solution that smells like rotten eggs when exposed to moist air. despite the fact that the solid state is yellow, the solution is colourless. Sodium sulfide (anhydrous) is a sulfide salt with formula na2s. the pentahydrate and (particularly) the nonahydrate are also known. in gel form, sodium sulfide is used to soften toenails to assist in trimming (and so relive pain) of ingrowing toenails. it is a sulfide salt and an inorganic sodium salt. chebi.

Sodium Sulphide Formula Explained With Preparation And Its Uses Sodium sulfide, or na2s, is an inorganic chemical compound with the formula of na2s that has risen to prominence in the organic chemical industry. it's a powerful alkaline solution that smells like rotten eggs when exposed to moist air. despite the fact that the solid state is yellow, the solution is colourless. Sodium sulfide (anhydrous) is a sulfide salt with formula na2s. the pentahydrate and (particularly) the nonahydrate are also known. in gel form, sodium sulfide is used to soften toenails to assist in trimming (and so relive pain) of ingrowing toenails. it is a sulfide salt and an inorganic sodium salt. chebi. The reaction often works poorly unless an excess of the nucleophile is used because the product thiol can undergo a second s n 2 reaction with alkyl halide to give a sulfide (r–s–rʹ) as by product. to circumvent this problem, thiourea, (nh 2) 2 c ═ s (nh 2) 2 c ═ s, is often used as the nucleophile in the preparation of a thiol from an. Sodium sulfide plays a crucial role in the kraft process used in the pulp and paper industry. in this process sodium hydroxide is used to break down the tough lignin in wood fibres, turning the wood into wood pulp. lignin removal is essential for the production of high quality paper products. 2. oxygen scavenger in water treatment.

Sodium Sulfide Na2s Structure Molecular Mass Chemical Properties The reaction often works poorly unless an excess of the nucleophile is used because the product thiol can undergo a second s n 2 reaction with alkyl halide to give a sulfide (r–s–rʹ) as by product. to circumvent this problem, thiourea, (nh 2) 2 c ═ s (nh 2) 2 c ═ s, is often used as the nucleophile in the preparation of a thiol from an. Sodium sulfide plays a crucial role in the kraft process used in the pulp and paper industry. in this process sodium hydroxide is used to break down the tough lignin in wood fibres, turning the wood into wood pulp. lignin removal is essential for the production of high quality paper products. 2. oxygen scavenger in water treatment.

Preparation Properties Of Sodium Sulfite Youtube

Comments are closed.