Reaction Of Acids With Metal Sulphides Sulphites Bisulphites L10

Reaction Of Acids With Metal Sulphides Sulphites Bisulphites L10 Acids bases and salts class 10th (cbsc,ncert,jkbose)lecture 10: reaction of acids with metal sulphides, sulphites & bisulphiteswhat is inside the video:* che. Sulphur dioxide gas is evolved usually when acids get reacted with sulphites and bisulphites.this video is about: reaction of acids with sulphites and bisulp.

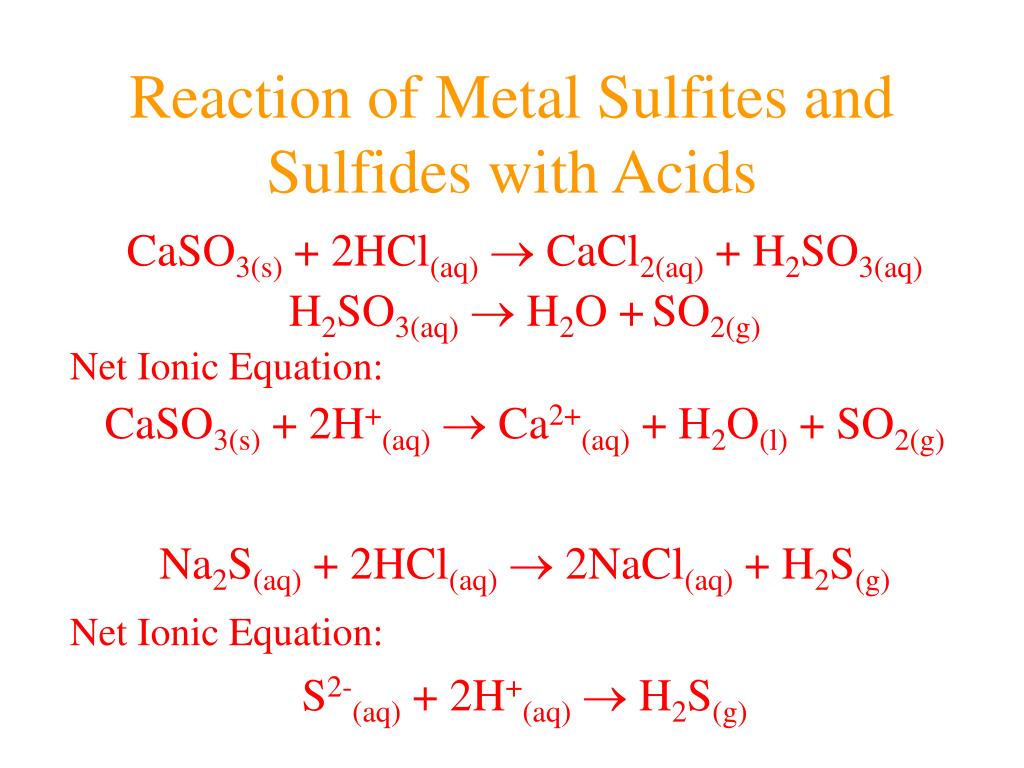
Reaction Of Acids With Metal Sulphite And Bisulphite Class 10 Here we will discuss about reaction of acids with metal sulphite and bisulphite and also its important questions related to this topic.for any further query. Predicting the extent of a reaction free. chapter 2 acid, bases & salts. chapter 3 organic chemistry. chapter 4 hydrocarbons. chapter 5 biochemistry. Acids and bases react with metals. acids react with most metals to form a salt and hydrogen gas. as discussed previously, metals that are more active than acids can undergo a single displacement reaction. for example, zinc metal reacts with hydrochloric acid, producing zinc chloride and hydrogen gas. zn(s) 2hcl(aq) → zncl2(aq) h2(g) zn (s. Solution. step 1) write the formula of acid and base in the reactants and salt and water in the products. all the strong electrolytes dissolve in water, so use (aq) to represent their state. h2so4(aq) ba(oh)2(aq) → salt(aq) h2o(l) step 2) balance the h in the acid with the oh in the base.
Ppt Acids And Bases Powerpoint Presentation Free Download Id 4503088 Acids and bases react with metals. acids react with most metals to form a salt and hydrogen gas. as discussed previously, metals that are more active than acids can undergo a single displacement reaction. for example, zinc metal reacts with hydrochloric acid, producing zinc chloride and hydrogen gas. zn(s) 2hcl(aq) → zncl2(aq) h2(g) zn (s. Solution. step 1) write the formula of acid and base in the reactants and salt and water in the products. all the strong electrolytes dissolve in water, so use (aq) to represent their state. h2so4(aq) ba(oh)2(aq) → salt(aq) h2o(l) step 2) balance the h in the acid with the oh in the base. What you should be comfortable with before you go on. metals above hydrogen in the reactivity series react with acids; those below hydrogen in the reactivity series don't. of the metals above hydrogen, reactivity increases the further up the reactivity series you go. a reaction with dilute sulfuric acid gives a metal sulfate and hydrogen. In more dilute acid, the rate picks up because there is more free rnh 2 in solution. dehydration of the aminoalkanol (equation 16 6) is acid catalyzed; this reaction normally is fast at ph values smaller than 3 4. therefore, the slow step at ph < 4 is addition of rnh 2 to the carbonyl group as per equation 16 5.

Reaction Of Acids With Sulphites And Bisulphites Chemistry Lecture What you should be comfortable with before you go on. metals above hydrogen in the reactivity series react with acids; those below hydrogen in the reactivity series don't. of the metals above hydrogen, reactivity increases the further up the reactivity series you go. a reaction with dilute sulfuric acid gives a metal sulfate and hydrogen. In more dilute acid, the rate picks up because there is more free rnh 2 in solution. dehydration of the aminoalkanol (equation 16 6) is acid catalyzed; this reaction normally is fast at ph values smaller than 3 4. therefore, the slow step at ph < 4 is addition of rnh 2 to the carbonyl group as per equation 16 5.

Reaction Of Acids With Sulphides Chemistry Lecture Sabaq Pk Youtube

Comments are closed.