Reaction Of Zinc With Dilute Sulphuric Acid Chemistry Experiment Grade 9

Reaction Of Zinc With Dilute Sulphuric Acid Chemistry Experim 1. zinc reacts with dilute sulphuric acid to produce hydrogen gas and zinc sulphate. zn h 2 so 4 → znso 4 h 2. 2. hydrogen gas burns with a pop sound. 3. it is a type of displacement reaction of a non metal by a metal. 4. the reaction is an example of chemical change. Procedure: add 10 ml of dilute sulphuric acid to a test tube containing a few granules of zinc metal. fit a rubber cork with a glass tube into the mouth of the test tube. observe the colour and the odour of the gas evolved in the reaction mixture. bring a lit candle near the opening of the glass tube in the mouth of the test tube.
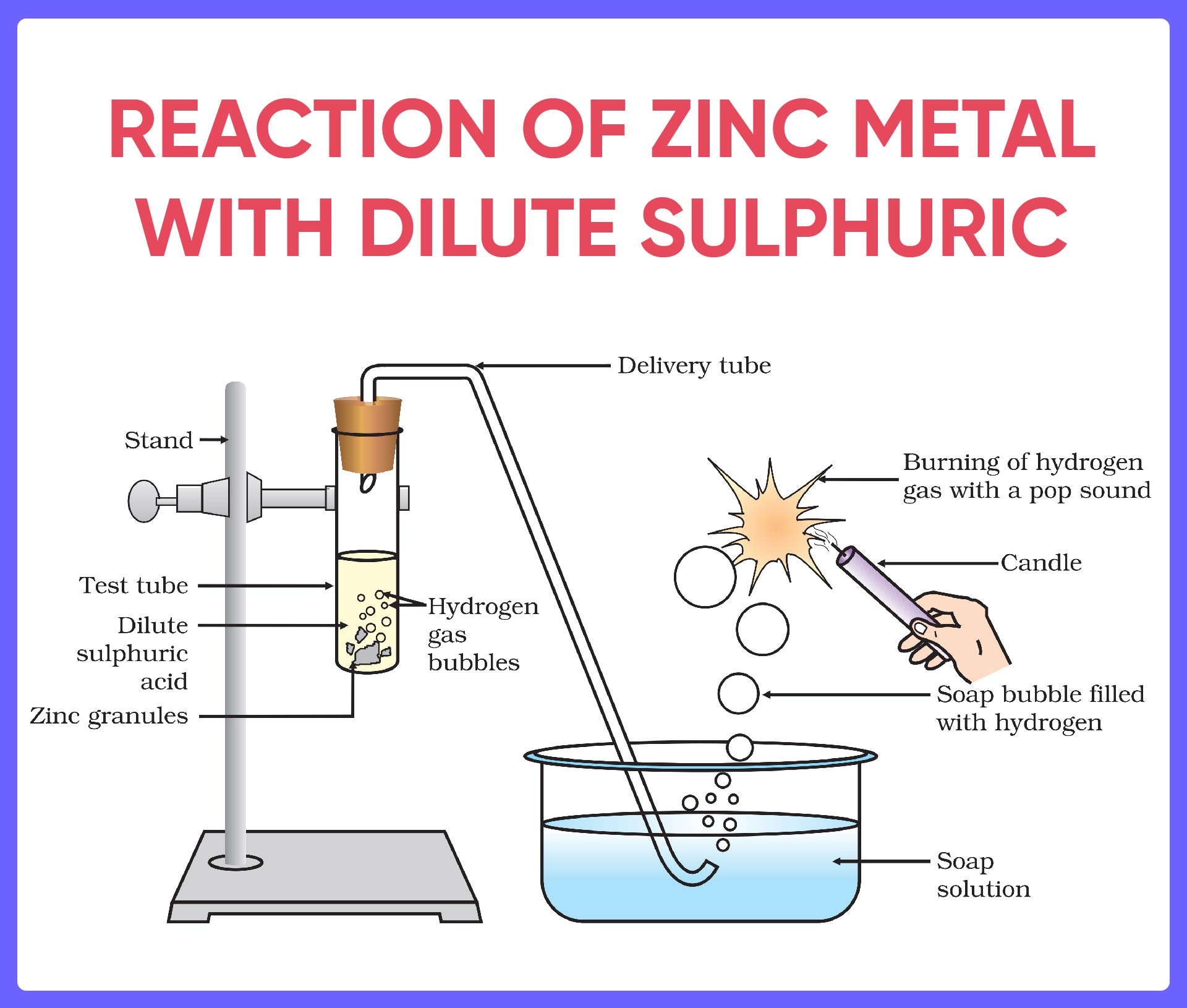
Reactions Of Acids And Bases Full List With Examples Teachoo Reaction of zinc with dilute sulphuric acid and classify as physical or chemical change | chemistry experiment | grade 9watch our other videos:english storie. Zinc reacts with dilute sulphuric acid to produce hydrogen gas (h 2) and zinc sulphate. this is an example of displacement reaction of a non metal by a metal. 2. zinc is more reactive than hydrogen. 4.znso 4 is different in chemical composition and chemical properties than zn and h 2 so 4 so it is a chemical change. 1. This video channel is developed by amrita university's create amrita.edu create for more information @ amrita.olabs.edu.in ?sub=73&brch=2&si. The reaction is: zinc sulfuric acid → zinc sulfate hydrogen. zn (s) h 2 so 4 (aq) → znso 4 (aq) h 2 (g) in test tube 2, copper is the catalyst for the reaction, and the reaction should be faster than in test tube 1, but may not be as fast as test tube 3. in test tube 3, zinc displaces copper from the copper sulfate solution and the.

Reaction Of Zinc Granules With Dilute Sulphuric Acid And Testing This video channel is developed by amrita university's create amrita.edu create for more information @ amrita.olabs.edu.in ?sub=73&brch=2&si. The reaction is: zinc sulfuric acid → zinc sulfate hydrogen. zn (s) h 2 so 4 (aq) → znso 4 (aq) h 2 (g) in test tube 2, copper is the catalyst for the reaction, and the reaction should be faster than in test tube 1, but may not be as fast as test tube 3. in test tube 3, zinc displaces copper from the copper sulfate solution and the. Apparatus for recovering salt formed by the reaction of zinc and sulfuric acid. measure 50 cm 3 of dilute sulfuric acid using a measuring cylinder and pour it into the beaker. warm this acid gently over a low, non smokey, bunsen flame. turn off the bunsen burner before the solution boils. Here is the detailed procedure for activity 2.3 from the class 10 science ncert book: zinc granule reaction with acid. setting up the apparatus: arrange the apparatus as shown in the figure given below. this includes a test tube, a delivery tube, a soap solution, and a candle. adding zinc to acid: pour about 5 ml of dilute sulphuric acid into.

Reaction Of Zinc With Dilute Hydrochloric Acid Or Sulphuric Acid Apparatus for recovering salt formed by the reaction of zinc and sulfuric acid. measure 50 cm 3 of dilute sulfuric acid using a measuring cylinder and pour it into the beaker. warm this acid gently over a low, non smokey, bunsen flame. turn off the bunsen burner before the solution boils. Here is the detailed procedure for activity 2.3 from the class 10 science ncert book: zinc granule reaction with acid. setting up the apparatus: arrange the apparatus as shown in the figure given below. this includes a test tube, a delivery tube, a soap solution, and a candle. adding zinc to acid: pour about 5 ml of dilute sulphuric acid into.

Cbse Class 9 Chemistry Practicals And Experiment On The Reaction Of

Comments are closed.